Create an account to track your scores
and create your own practice tests:
Test: GRE Subject Test: Chemistry
| 1. | Which of the following is true regarding the solubility product constant, |
The concentration of substance 
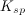
As more of substance 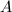


Roldan
Certified Tutor
Certified Tutor
Barry University, Bachelor of Chemistry, Chemistry. New York Chiropractic College, Master of Science, Anatomy.
Popular Subjects
ACT Tutors in Boston, LSAT Tutors in San Francisco-Bay Area, Biology Tutors in Philadelphia, SAT Tutors in Denver, French Tutors in Washington DC, Computer Science Tutors in San Diego, Statistics Tutors in Denver, LSAT Tutors in Dallas Fort Worth, English Tutors in Los Angeles, English Tutors in Philadelphia
Popular Courses & Classes
ACT Courses & Classes in Boston, LSAT Courses & Classes in Boston, GMAT Courses & Classes in Boston, ACT Courses & Classes in Philadelphia, GMAT Courses & Classes in San Francisco-Bay Area, GRE Courses & Classes in Boston, ISEE Courses & Classes in Miami, Spanish Courses & Classes in Seattle, ACT Courses & Classes in Washington DC, Spanish Courses & Classes in Washington DC
Popular Test Prep
GMAT Test Prep in Phoenix, GRE Test Prep in San Francisco-Bay Area, MCAT Test Prep in Chicago, SAT Test Prep in Houston, MCAT Test Prep in Miami, GMAT Test Prep in Boston, GMAT Test Prep in San Francisco-Bay Area, ACT Test Prep in Washington DC, SSAT Test Prep in Seattle, ACT Test Prep in Boston

 , for a reaction in the form:
, for a reaction in the form:
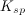
![K_{sp}=[C]^c[D]^d](https://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/73718/gif.latex)



