Create an account to track your scores
and create your own practice tests:
Test: College Chemistry
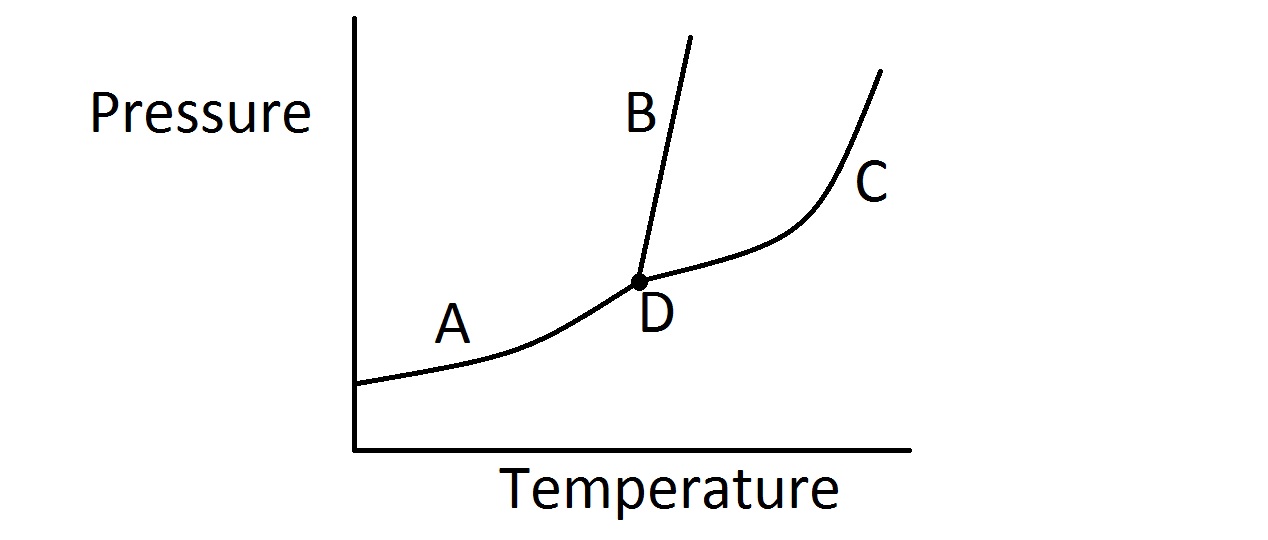
| 1. | Consider the typical phase diagram of a compound given below.
Which of the following lines or points on the diagram represents a situation in which the rate of vaporization of the compound is equal to its rate of condensation? |
Line A
Line B
Line C
Point D
None of these
Popular Subjects
Physics Tutors in Phoenix, Chemistry Tutors in Miami, Calculus Tutors in Washington DC, Chemistry Tutors in Houston, ISEE Tutors in Miami, Reading Tutors in San Diego, Math Tutors in San Francisco-Bay Area, SSAT Tutors in Seattle, Calculus Tutors in Dallas Fort Worth, GRE Tutors in Miami
Popular Courses & Classes
ISEE Courses & Classes in New York City, SSAT Courses & Classes in Seattle, SSAT Courses & Classes in Washington DC, GMAT Courses & Classes in Boston, LSAT Courses & Classes in Houston, ACT Courses & Classes in San Diego, SSAT Courses & Classes in Los Angeles, GMAT Courses & Classes in San Francisco-Bay Area, Spanish Courses & Classes in Washington DC, MCAT Courses & Classes in Dallas Fort Worth
Popular Test Prep
GRE Test Prep in San Francisco-Bay Area, GMAT Test Prep in Phoenix, MCAT Test Prep in Phoenix, SAT Test Prep in Chicago, GMAT Test Prep in Denver, ACT Test Prep in San Diego, MCAT Test Prep in Philadelphia, LSAT Test Prep in Los Angeles, MCAT Test Prep in Dallas Fort Worth, SAT Test Prep in Phoenix