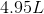Create an account to track your scores
and create your own practice tests:
Test: AP Chemistry
| 1. | Consider the reaction of baking soda and vinegar in an aqueous solution: If we begin with 17g of Assume that all gases behave as ideal gases at atmospheric pressure. The temperature is 298K. |
Emily
Certified Tutor
Certified Tutor
University of Pennsylvania, Bachelor of Science, Chemical and Biomolecular Engineering.
Popular Subjects
Biology Tutors in Philadelphia, Physics Tutors in Boston, English Tutors in Phoenix, French Tutors in Seattle, French Tutors in Atlanta, Reading Tutors in Seattle, GRE Tutors in Atlanta, Algebra Tutors in Seattle, ISEE Tutors in New York City, ISEE Tutors in Boston
Popular Courses & Classes
GMAT Courses & Classes in San Francisco-Bay Area, ACT Courses & Classes in Phoenix, SAT Courses & Classes in Boston, Spanish Courses & Classes in San Diego, SAT Courses & Classes in New York City, SSAT Courses & Classes in Boston, SSAT Courses & Classes in New York City, ACT Courses & Classes in Philadelphia, GMAT Courses & Classes in Boston, GRE Courses & Classes in Los Angeles
Popular Test Prep
SAT Test Prep in Los Angeles, SSAT Test Prep in Atlanta, GMAT Test Prep in Washington DC, ISEE Test Prep in Miami, GRE Test Prep in San Diego, GMAT Test Prep in Boston, SSAT Test Prep in Chicago, SAT Test Prep in Phoenix, MCAT Test Prep in Houston, GMAT Test Prep in San Francisco-Bay Area


 and excess
and excess  , what is the volume of the gas released?
, what is the volume of the gas released?