Molecular and Electronic Geometries
Help Questions
AP Chemistry › Molecular and Electronic Geometries
Questions 1 - 1
1
What is the molecular geometry of 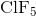
Square pyramidal
Square planar
Octahedral
Trigonal pyramidal
Tetrahedral
Explanation
There are six electron domains in 


AI TutorYour personal study buddy
Question of the DayDaily practice to build your skills
FlashcardsStudy and memorize key concepts
WorksheetsBuild custom practice quizzes
AI SolverStep-by-step problem solutions
Learn by ConceptMaster concepts step by step
Diagnostic TestsAssess your knowledge level
Practice TestsTest your skills with exam questions
GamesLearn through free games