VSEPR Theory
Help Questions
AP Chemistry › VSEPR Theory
Questions 1 - 1
1
Based on VSEPR theory and electron configurations, which of the following molecules is not matched with its correct geometry?



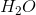

Explanation

AI TutorYour personal study buddy
Question of the DayDaily practice to build your skills
FlashcardsStudy and memorize key concepts
WorksheetsBuild custom practice quizzes
AI SolverStep-by-step problem solutions
Learn by ConceptMaster concepts step by step
Diagnostic TestsAssess your knowledge level
Practice TestsTest your skills with exam questions
GamesLearn through free games