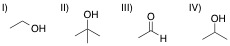Create an account to track your scores
and create your own practice tests:
Test: Organic Chemistry
| 1. | A chemist adds the orange oxidizing agent, Na2Cr2O7, to the following substrates and dissolves the mixture in an aqueous solution of sulfuric acid. Oxidation is indicated by the disappearance of the orange color. Which of the substrate-oxidant solutions will remain orange? |
I
IV
II
All of the solutions will lose their orange color, indicating oxidation
III
Danielle
Certified Tutor
Certified Tutor
University of Illinois at Urbana-Champaign, Bachelor of Science, Chemistry. University of Illinois at Chicago, Doctor of Phil...
Organic Chemistry Tutors in Top Cities:
Atlanta Organic Chemistry Tutors, Austin Organic Chemistry Tutors, Boston Organic Chemistry Tutors, Chicago Organic Chemistry Tutors, Dallas Fort Worth Organic Chemistry Tutors, Denver Organic Chemistry Tutors, Houston Organic Chemistry Tutors, Kansas City Organic Chemistry Tutors, Los Angeles Organic Chemistry Tutors, Miami Organic Chemistry Tutors, New York City Organic Chemistry Tutors, Philadelphia Organic Chemistry Tutors, Phoenix Organic Chemistry Tutors, San Diego Organic Chemistry Tutors, San Francisco-Bay Area Organic Chemistry Tutors, Seattle Organic Chemistry Tutors, St. Louis Organic Chemistry Tutors, Tucson Organic Chemistry Tutors, Washington DC Organic Chemistry Tutors
Popular Courses & Classes
ISEE Courses & Classes in Miami, GRE Courses & Classes in New York City, GRE Courses & Classes in Miami, Spanish Courses & Classes in Chicago, MCAT Courses & Classes in Atlanta, LSAT Courses & Classes in Philadelphia, SSAT Courses & Classes in Philadelphia, SSAT Courses & Classes in Washington DC, MCAT Courses & Classes in Chicago, MCAT Courses & Classes in Philadelphia
Popular Test Prep
SSAT Test Prep in Los Angeles, ACT Test Prep in San Francisco-Bay Area, ISEE Test Prep in Miami, ACT Test Prep in Denver, ACT Test Prep in New York City, SSAT Test Prep in Miami, SAT Test Prep in New York City, GRE Test Prep in New York City, LSAT Test Prep in Denver, LSAT Test Prep in Philadelphia