All SAT II Chemistry Resources
Example Questions
Example Question #32 : Sat Subject Test In Chemistry
Suppose that an ideal gas at 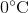

Possible Answers:
Correct answer:
Explanation:
First of all, the unit degrees Celsius should be converted to Kelvins by adding 273. This means that the temperature increases from 273 K to 323 K.
Next, the combined gas law can be used.
Plugging in the known values gives

and solving for the volume gives
Rearranging shows this is clearly equivalent to the correct answer, 
Brianna
Certified Tutor
Certified Tutor
Fairfield University, Bachelor of Science, Biology, General. National University of Ireland at Galway, Master of Science, Neu...
Aamilah
Certified Tutor
Certified Tutor
Johns Hopkins University, Master of Science, Biomedical Engineering. Johns Hopkins University, Bachelor of Science, Biomedica...
All SAT II Chemistry Resources
Popular Subjects
GMAT Tutors in Seattle, Math Tutors in Houston, MCAT Tutors in Chicago, Chemistry Tutors in Dallas Fort Worth, ACT Tutors in Los Angeles, Algebra Tutors in Houston, Chemistry Tutors in Houston, Calculus Tutors in Denver, Reading Tutors in Phoenix, SSAT Tutors in Los Angeles
Popular Courses & Classes
GMAT Courses & Classes in New York City, SSAT Courses & Classes in Phoenix, Spanish Courses & Classes in Miami, GMAT Courses & Classes in San Diego, ISEE Courses & Classes in Boston, ACT Courses & Classes in San Francisco-Bay Area, LSAT Courses & Classes in Denver, ISEE Courses & Classes in Philadelphia, Spanish Courses & Classes in San Diego, ACT Courses & Classes in Denver
Popular Test Prep
SAT Test Prep in San Francisco-Bay Area, GMAT Test Prep in San Diego, SSAT Test Prep in Chicago, GMAT Test Prep in New York City, ACT Test Prep in San Francisco-Bay Area, MCAT Test Prep in Seattle, SSAT Test Prep in Dallas Fort Worth, SSAT Test Prep in Washington DC, ACT Test Prep in Philadelphia, SAT Test Prep in Miami











