Organic Oxidizing Agents - Organic Chemistry
Card 1 of 68
What is the product when 2-butanol is treated with PCC?
What is the product when 2-butanol is treated with PCC?
Tap to reveal answer
PCC is an oxidizing agent. It converts alcohols to ketones, but is not strong enough to convert primary alcohols to carboxylic acids. 2-butanol has a hydroxy group on its carbon 2. The addition of PCC will convert this hydroxy group into a carbonyl, producing 2-butanone.
PCC is an oxidizing agent. It converts alcohols to ketones, but is not strong enough to convert primary alcohols to carboxylic acids. 2-butanol has a hydroxy group on its carbon 2. The addition of PCC will convert this hydroxy group into a carbonyl, producing 2-butanone.
← Didn't Know|Knew It →
What is the product of 1-pentanol when it is treated with PCC?
What is the product of 1-pentanol when it is treated with PCC?
Tap to reveal answer
PCC is an oxidizing agent. It converts alcohols to carbonyls, but is not strong enough to convert a primary alcohol into a carboxylic acid. It only converts primary alcohols to aldehydes, and secondary alcohols to ketones. 1-pentanol is a primary alcohol so it will be converted to the aldehyde pentanal.
PCC is an oxidizing agent. It converts alcohols to carbonyls, but is not strong enough to convert a primary alcohol into a carboxylic acid. It only converts primary alcohols to aldehydes, and secondary alcohols to ketones. 1-pentanol is a primary alcohol so it will be converted to the aldehyde pentanal.
← Didn't Know|Knew It →

Which reagent is best-suited to accomplish the given reaction?

Which reagent is best-suited to accomplish the given reaction?
Tap to reveal answer
PCC is an oxidizing agent that reacts with primary and secondary alcohols. However, it is less reactive than potassium permanganate and chromic acid. PCC differs from chromic acid by oxidizing primary alcohols to aldehydes, whereas chromic acid oxidizes primary alcohols and aldehydes to carboxylic acids. The desired product of the reaction given requires that the primary alcohol be oxidized to an aldehyde, so PCC is the best option.  is a reducing agent and would have the opposite effect than what is desired, yielding an alkane.
is a reducing agent and would have the opposite effect than what is desired, yielding an alkane.
PCC is an oxidizing agent that reacts with primary and secondary alcohols. However, it is less reactive than potassium permanganate and chromic acid. PCC differs from chromic acid by oxidizing primary alcohols to aldehydes, whereas chromic acid oxidizes primary alcohols and aldehydes to carboxylic acids. The desired product of the reaction given requires that the primary alcohol be oxidized to an aldehyde, so PCC is the best option. 
← Didn't Know|Knew It →

Which reagents are required to drive the given reaction?
Which reagents are required to drive the given reaction?
Tap to reveal answer
This is a two step reaction. In the first step, an alcohol is substituted for the bromine via an  reaction. Next, the alcohol is oxidized into a ketone with
reaction. Next, the alcohol is oxidized into a ketone with  , a strong oxidizing agent used almost exclusively for converting alcohols into carbonyls.
, a strong oxidizing agent used almost exclusively for converting alcohols into carbonyls.
This is a two step reaction. In the first step, an alcohol is substituted for the bromine via an 

← Didn't Know|Knew It →
Which of the following is not true regarding the reagent  ?
?
Which of the following is not true regarding the reagent 
Tap to reveal answer
 has the capability of oxidizing primary alcohols into aldehydes and secondary alcohols into ketones. However, it cannot oxidize aldehydes into carboxylic acids. To do that, we would need a stronger oxidizing agent such as
has the capability of oxidizing primary alcohols into aldehydes and secondary alcohols into ketones. However, it cannot oxidize aldehydes into carboxylic acids. To do that, we would need a stronger oxidizing agent such as  .
.


← Didn't Know|Knew It →
What is the product of the reaction shown?


What is the product of the reaction shown?


Tap to reveal answer
First step: PCC oxidizes the primary alcohol to acetaldehyde
Second step: Grignard reagent attacks carbonyl carbon
Third step: Neutralization of the anion forms isoproyl alcohol
First step: PCC oxidizes the primary alcohol to acetaldehyde
Second step: Grignard reagent attacks carbonyl carbon
Third step: Neutralization of the anion forms isoproyl alcohol
← Didn't Know|Knew It →
Which of the following compounds is not a reducing agent?
Which of the following compounds is not a reducing agent?
Tap to reveal answer
 is the only compound listed that is not a reducing agent. Pyridinium chlorochromate is a weak oxidizing agent and is often used to oxidize alcohols into carbony compounds. All of the other compounds are similar in that they function as reducing agents.
is the only compound listed that is not a reducing agent. Pyridinium chlorochromate is a weak oxidizing agent and is often used to oxidize alcohols into carbony compounds. All of the other compounds are similar in that they function as reducing agents.

← Didn't Know|Knew It →

What would be the product of the given reaction?

What would be the product of the given reaction?
Tap to reveal answer
The reaction given would give an aldehyde. This type of reaction is called an oxidation reaction. Oxidation of a primary alcohol as in the reaction given by PCC (pyridinium chlorochromate) in  (dichloromethane) solvent yields an aldehyde. Like chromic acid, PCC oxidizes alcohols. However, PCC only oxidizes primary alcohols one step up to aldehydes and secondary alcohols to ketones. Chromic acid is a harsher oxidant because it will oxidize aldehydes to carboxylic acids. Below is the mechanism for this reaction:
(dichloromethane) solvent yields an aldehyde. Like chromic acid, PCC oxidizes alcohols. However, PCC only oxidizes primary alcohols one step up to aldehydes and secondary alcohols to ketones. Chromic acid is a harsher oxidant because it will oxidize aldehydes to carboxylic acids. Below is the mechanism for this reaction:

The reaction given would give an aldehyde. This type of reaction is called an oxidation reaction. Oxidation of a primary alcohol as in the reaction given by PCC (pyridinium chlorochromate) in 

← Didn't Know|Knew It →

What would be the product of the given reaction?

What would be the product of the given reaction?
Tap to reveal answer
The reaction given would give an aldehyde. This type of reaction is called an oxidation reaction. Oxidation of a primary alcohol as in the reaction given by PCC (pyridinium chlorochromate) in  (dichloromethane) solvent yields an aldehyde. Like chromic acid, PCC oxidizes alcohols. However, PCC only oxidizes primary alcohols one step up to aldehydes and secondary alcohols to ketones. Chromic acid is a harsher oxidant because it will oxidize aldehydes to carboxylic acids. Below is the mechanism for this reaction: Below is the mechanism:
(dichloromethane) solvent yields an aldehyde. Like chromic acid, PCC oxidizes alcohols. However, PCC only oxidizes primary alcohols one step up to aldehydes and secondary alcohols to ketones. Chromic acid is a harsher oxidant because it will oxidize aldehydes to carboxylic acids. Below is the mechanism for this reaction: Below is the mechanism:

The reaction given would give an aldehyde. This type of reaction is called an oxidation reaction. Oxidation of a primary alcohol as in the reaction given by PCC (pyridinium chlorochromate) in 

← Didn't Know|Knew It →
What is the product of the reaction shown?

What is the product of the reaction shown?
Tap to reveal answer
PCC can be used to oxidize primary alcohols into aldehydes, or secondary alcohols into ketones. The starting material shown is a secondary alcohol, so the product will be a ketone (a carbonyl ( ) group where the carbonyl carbon is also attached to two other carbons).
) group where the carbonyl carbon is also attached to two other carbons).
PCC can be used to oxidize primary alcohols into aldehydes, or secondary alcohols into ketones. The starting material shown is a secondary alcohol, so the product will be a ketone (a carbonyl (
← Didn't Know|Knew It →
A chemist adds the orange oxidizing agent, Na2Cr2O7, to the following substrates and dissolves the mixture in an aqueous solution of sulfuric acid. Oxidation is indicated by the disappearance of the orange color. Which of the substrate-oxidant solutions will remain orange?
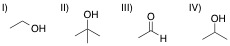
A chemist adds the orange oxidizing agent, Na2Cr2O7, to the following substrates and dissolves the mixture in an aqueous solution of sulfuric acid. Oxidation is indicated by the disappearance of the orange color. Which of the substrate-oxidant solutions will remain orange?
Tap to reveal answer
The following reaction schemes show the oxidation of all substrates, indicating that substrate II is in the highest oxidation state possible, and that an oxidation of this compound will not proceed.
Remember that in sulfuric acid and water, Na2Cr2O7 will be converted to CrO3, the active oxidant species. Furthermore, the oxidation mechanism involving this species includes the key step in which a hydrogen bonded to the carbon in question is eliminated_,_ and simutaneously, a double bond from that carbon to an oxygen is installed. Thus, all substrates that feature at least one hydrogen bonded to the carbon to be oxidized can and will be oxidized in the precense of chromium trioxide.
Lastly, remember that these reactions are taking place in the prescence of water. While substrates such as compound III do not appear to be oxidizable, attack of water at the aldehyde carbon will give a dialcohol tetrathedral intermediate that can be immediately oxidized by chromium trioxide to the corresponding carboxylic acid. A similar mechanism occurs for substrate I, wherein, after the ketone oxidation state is achieved, an attack of water furnishes the same dialcohol intermediate that is oxidized to the carboxylic acid. Remember that the highest oxidation state available for organic compounds containing more than one carbon is the carboxylic acid oxidation state. Chromium trioxide will oxidize all organics to this oxidation state, unless directly-bonded hydrogens are not present in lower oxidation states, such as shown with substrate IV.

The following reaction schemes show the oxidation of all substrates, indicating that substrate II is in the highest oxidation state possible, and that an oxidation of this compound will not proceed.
Remember that in sulfuric acid and water, Na2Cr2O7 will be converted to CrO3, the active oxidant species. Furthermore, the oxidation mechanism involving this species includes the key step in which a hydrogen bonded to the carbon in question is eliminated_,_ and simutaneously, a double bond from that carbon to an oxygen is installed. Thus, all substrates that feature at least one hydrogen bonded to the carbon to be oxidized can and will be oxidized in the precense of chromium trioxide.
Lastly, remember that these reactions are taking place in the prescence of water. While substrates such as compound III do not appear to be oxidizable, attack of water at the aldehyde carbon will give a dialcohol tetrathedral intermediate that can be immediately oxidized by chromium trioxide to the corresponding carboxylic acid. A similar mechanism occurs for substrate I, wherein, after the ketone oxidation state is achieved, an attack of water furnishes the same dialcohol intermediate that is oxidized to the carboxylic acid. Remember that the highest oxidation state available for organic compounds containing more than one carbon is the carboxylic acid oxidation state. Chromium trioxide will oxidize all organics to this oxidation state, unless directly-bonded hydrogens are not present in lower oxidation states, such as shown with substrate IV.

← Didn't Know|Knew It →

Which of the following reagents would satisfy the given reaction?
Which of the following reagents would satisfy the given reaction?
Tap to reveal answer
In order to drive the reactant, we must first convert the alcohol group on the ethanol into a carboxylic acid. We do so by using the oxidizing agent,  , a very strong oxidizing agent that is well known to oxidize primary alcohols into carboxylic acids (among other functions). Once we have our carboxylic acid, we can simply use
, a very strong oxidizing agent that is well known to oxidize primary alcohols into carboxylic acids (among other functions). Once we have our carboxylic acid, we can simply use  to convert our carboxylic acid into an acid halide to attain our desired final product.
to convert our carboxylic acid into an acid halide to attain our desired final product.
In order to drive the reactant, we must first convert the alcohol group on the ethanol into a carboxylic acid. We do so by using the oxidizing agent, 

← Didn't Know|Knew It →
Which of the following substrates will be oxidized into a ketone when reacting with 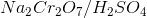 ?
?
Which of the following substrates will be oxidized into a ketone when reacting with 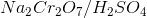
Tap to reveal answer
 is a strong oxidizing agent.
is a strong oxidizing agent.
Not only can  reduce secondary alcohols into ketones, but it can reduce primary alcohols and aldehydes into carboxylic acids.
reduce secondary alcohols into ketones, but it can reduce primary alcohols and aldehydes into carboxylic acids.

Not only can 
← Didn't Know|Knew It →

What would be the product of the given reaction?

What would be the product of the given reaction?
Tap to reveal answer
The reaction given would give a ketone. This type of reaction is called an oxidation reaction. Oxidation of a secondary alcohol as in the reaction given by  (sodium dichromate) in an aqueous solution of
(sodium dichromate) in an aqueous solution of  (acetic acid) solvent yields a ketone. However, if we performed the same reaction with a primary alcohol, a carboxylic acid would have formed.
(acetic acid) solvent yields a ketone. However, if we performed the same reaction with a primary alcohol, a carboxylic acid would have formed.
The reaction given would give a ketone. This type of reaction is called an oxidation reaction. Oxidation of a secondary alcohol as in the reaction given by 

← Didn't Know|Knew It →
What is the product when 1-propanol is treated with potassium permanganate?
What is the product when 1-propanol is treated with potassium permanganate?
Tap to reveal answer
Potassium permanganate is a strong oxidizing agent. It can convert secondary alcohols to ketones. It can also convert primary alcohols to carboxylic acids. 1-propanol has a hydroxy group on carbon 1, so it is primary; thus it will be converted to propanoic acid.
Potassium permanganate is a strong oxidizing agent. It can convert secondary alcohols to ketones. It can also convert primary alcohols to carboxylic acids. 1-propanol has a hydroxy group on carbon 1, so it is primary; thus it will be converted to propanoic acid.
← Didn't Know|Knew It →

Which of the following reagents would satisfy the given reaction?
Which of the following reagents would satisfy the given reaction?
Tap to reveal answer
In order to drive the reactant, we must first convert the alcohol group on the ethanol into a carboxylic acid. We do so by using the oxidizing agent,  , a very strong oxidizing agent that is well known to oxidize primary alcohols into carboxylic acids (among other functions). Once we have our carboxylic acid, we can simply use
, a very strong oxidizing agent that is well known to oxidize primary alcohols into carboxylic acids (among other functions). Once we have our carboxylic acid, we can simply use  to convert our carboxylic acid into an acid halide to attain our desired final product.
to convert our carboxylic acid into an acid halide to attain our desired final product.
In order to drive the reactant, we must first convert the alcohol group on the ethanol into a carboxylic acid. We do so by using the oxidizing agent, 

← Didn't Know|Knew It →
Which of the following substrates will be oxidized into a ketone when reacting with 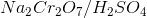 ?
?
Which of the following substrates will be oxidized into a ketone when reacting with 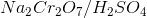
Tap to reveal answer
 is a strong oxidizing agent.
is a strong oxidizing agent.
Not only can  reduce secondary alcohols into ketones, but it can reduce primary alcohols and aldehydes into carboxylic acids.
reduce secondary alcohols into ketones, but it can reduce primary alcohols and aldehydes into carboxylic acids.

Not only can 
← Didn't Know|Knew It →
A chemist adds the orange oxidizing agent, Na2Cr2O7, to the following substrates and dissolves the mixture in an aqueous solution of sulfuric acid. Oxidation is indicated by the disappearance of the orange color. Which of the substrate-oxidant solutions will remain orange?
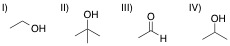
A chemist adds the orange oxidizing agent, Na2Cr2O7, to the following substrates and dissolves the mixture in an aqueous solution of sulfuric acid. Oxidation is indicated by the disappearance of the orange color. Which of the substrate-oxidant solutions will remain orange?
Tap to reveal answer
The following reaction schemes show the oxidation of all substrates, indicating that substrate II is in the highest oxidation state possible, and that an oxidation of this compound will not proceed.
Remember that in sulfuric acid and water, Na2Cr2O7 will be converted to CrO3, the active oxidant species. Furthermore, the oxidation mechanism involving this species includes the key step in which a hydrogen bonded to the carbon in question is eliminated_,_ and simutaneously, a double bond from that carbon to an oxygen is installed. Thus, all substrates that feature at least one hydrogen bonded to the carbon to be oxidized can and will be oxidized in the precense of chromium trioxide.
Lastly, remember that these reactions are taking place in the prescence of water. While substrates such as compound III do not appear to be oxidizable, attack of water at the aldehyde carbon will give a dialcohol tetrathedral intermediate that can be immediately oxidized by chromium trioxide to the corresponding carboxylic acid. A similar mechanism occurs for substrate I, wherein, after the ketone oxidation state is achieved, an attack of water furnishes the same dialcohol intermediate that is oxidized to the carboxylic acid. Remember that the highest oxidation state available for organic compounds containing more than one carbon is the carboxylic acid oxidation state. Chromium trioxide will oxidize all organics to this oxidation state, unless directly-bonded hydrogens are not present in lower oxidation states, such as shown with substrate IV.

The following reaction schemes show the oxidation of all substrates, indicating that substrate II is in the highest oxidation state possible, and that an oxidation of this compound will not proceed.
Remember that in sulfuric acid and water, Na2Cr2O7 will be converted to CrO3, the active oxidant species. Furthermore, the oxidation mechanism involving this species includes the key step in which a hydrogen bonded to the carbon in question is eliminated_,_ and simutaneously, a double bond from that carbon to an oxygen is installed. Thus, all substrates that feature at least one hydrogen bonded to the carbon to be oxidized can and will be oxidized in the precense of chromium trioxide.
Lastly, remember that these reactions are taking place in the prescence of water. While substrates such as compound III do not appear to be oxidizable, attack of water at the aldehyde carbon will give a dialcohol tetrathedral intermediate that can be immediately oxidized by chromium trioxide to the corresponding carboxylic acid. A similar mechanism occurs for substrate I, wherein, after the ketone oxidation state is achieved, an attack of water furnishes the same dialcohol intermediate that is oxidized to the carboxylic acid. Remember that the highest oxidation state available for organic compounds containing more than one carbon is the carboxylic acid oxidation state. Chromium trioxide will oxidize all organics to this oxidation state, unless directly-bonded hydrogens are not present in lower oxidation states, such as shown with substrate IV.

← Didn't Know|Knew It →

What would be the product of the given reaction?

What would be the product of the given reaction?
Tap to reveal answer
The reaction given would give a ketone. This type of reaction is called an oxidation reaction. Oxidation of a secondary alcohol as in the reaction given by  (sodium dichromate) in an aqueous solution of
(sodium dichromate) in an aqueous solution of  (acetic acid) solvent yields a ketone. However, if we performed the same reaction with a primary alcohol, a carboxylic acid would have formed.
(acetic acid) solvent yields a ketone. However, if we performed the same reaction with a primary alcohol, a carboxylic acid would have formed.
The reaction given would give a ketone. This type of reaction is called an oxidation reaction. Oxidation of a secondary alcohol as in the reaction given by 

← Didn't Know|Knew It →
What is the product when 1-propanol is treated with potassium permanganate?
What is the product when 1-propanol is treated with potassium permanganate?
Tap to reveal answer
Potassium permanganate is a strong oxidizing agent. It can convert secondary alcohols to ketones. It can also convert primary alcohols to carboxylic acids. 1-propanol has a hydroxy group on carbon 1, so it is primary; thus it will be converted to propanoic acid.
Potassium permanganate is a strong oxidizing agent. It can convert secondary alcohols to ketones. It can also convert primary alcohols to carboxylic acids. 1-propanol has a hydroxy group on carbon 1, so it is primary; thus it will be converted to propanoic acid.
← Didn't Know|Knew It →