0%
0 / 2 answered
Gibbs Free Energy Practice Test
•2 QuestionsQuestion
1 / 2
Q1
For constant temperature, Gibbs free energy is defined as:

Where ,  is the change in Gibbs free energy,
is the change in Gibbs free energy,  is the change in enthalpy,
is the change in enthalpy,  is temperature, and
is temperature, and 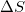 is the change in entropy.
is the change in entropy.
Given that a system is spontaneous, which of the following states are possible?
I. 
II. 
III. 
IV. 
For constant temperature, Gibbs free energy is defined as:
Where , 


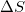
Given that a system is spontaneous, which of the following states are possible?
I.
II.
III.
IV.

 is the change in Gibbs Free Energy,
is the change in Gibbs Free Energy,  is the change in enthalpy,
is the change in enthalpy,  is temperature, and
is temperature, and  is the change in entropy.
is the change in entropy.