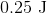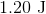Thermochemistry and Thermodynamics
Help Questions
Physical Chemistry › Thermochemistry and Thermodynamics
A sample of an ideal gas is initially at a volume of 



Explanation
The expression for the relationship between heat (


The work (


Plugging work (
Plugging the values given into the internal energy equation gives:
A sample of an ideal gas is initially at a volume of 



Explanation
The expression for the relationship between heat (


The work (


Plugging work (
Plugging the values given into the internal energy equation gives:
A sample of an ideal gas is initially at a volume of 



Explanation
The expression for the relationship between heat (


The work (


Plugging work (
Plugging the values given into the internal energy equation gives:
In an reaction, the products are more stable than the reactants; in an reaction the reactants are more stable than the products.
exergonic . . . endergonic
endergonic . . . exergonic
endergonic . . . endergonic
exergonic . . . exergonic
Explanation
Exergonic reactions release energy; therefore, the energy of products is lower than that of the reactants. Endergonic reactions consume energy; therefore, the energy of products is greater than that of the reactants. In other words, exergonic reactions are spontaneous, while endergonic reactions are nonspontaneous, and require the net input of energy to drive the reaction.
In an reaction, the products are more stable than the reactants; in an reaction the reactants are more stable than the products.
exergonic . . . endergonic
endergonic . . . exergonic
endergonic . . . endergonic
exergonic . . . exergonic
Explanation
Exergonic reactions release energy; therefore, the energy of products is lower than that of the reactants. Endergonic reactions consume energy; therefore, the energy of products is greater than that of the reactants. In other words, exergonic reactions are spontaneous, while endergonic reactions are nonspontaneous, and require the net input of energy to drive the reaction.
In an reaction, the products are more stable than the reactants; in an reaction the reactants are more stable than the products.
exergonic . . . endergonic
endergonic . . . exergonic
endergonic . . . endergonic
exergonic . . . exergonic
Explanation
Exergonic reactions release energy; therefore, the energy of products is lower than that of the reactants. Endergonic reactions consume energy; therefore, the energy of products is greater than that of the reactants. In other words, exergonic reactions are spontaneous, while endergonic reactions are nonspontaneous, and require the net input of energy to drive the reaction.
In an exergonic reaction, products will have Gibbs free energy and the reaction is .
lower . . . spontaneous
lower . . . nonspontaneous
higher . . . spontaneous
higher . . . nonspontaneous
Explanation
Exergonic reaction suggests that the Gibbs free energy is negative. Since the change in Gibbs free energy is defined as Gibbs free energy of products - Gibbs free energy of reactants, a negative change in Gibbs free energy suggests that the products have a lower Gibbs free energy than reactants. A reaction is spontaneous if it has negative Gibbs free energy; therefore, exergonic reactions are always spontaneous. This is because the reaction is producing a more stable product (lower energy) from a less stable reactant (higher energy).
At 



Explanation
The process as described by the question is an isothermal process. An isothermal process has a constant temperature, therefore, there is no change in temperature. For an isothermal reversible process, the work done by the system is:
Plugging the values given into the equation gives:
At 



Explanation
The process as described by the question is an isothermal process. An isothermal process has a constant temperature, therefore, there is no change in temperature. For an isothermal reversible process, the work done by the system is:
Plugging the values given into the equation gives:
In an exergonic reaction, products will have Gibbs free energy and the reaction is .
lower . . . spontaneous
lower . . . nonspontaneous
higher . . . spontaneous
higher . . . nonspontaneous
Explanation
Exergonic reaction suggests that the Gibbs free energy is negative. Since the change in Gibbs free energy is defined as Gibbs free energy of products - Gibbs free energy of reactants, a negative change in Gibbs free energy suggests that the products have a lower Gibbs free energy than reactants. A reaction is spontaneous if it has negative Gibbs free energy; therefore, exergonic reactions are always spontaneous. This is because the reaction is producing a more stable product (lower energy) from a less stable reactant (higher energy).