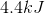Phase Changes
Help Questions
Physical Chemistry › Phase Changes
Questions 1 - 1
1
What amount of heat must be added to a 20-gram sample of ice in order to raise the temperature from 

Explanation
This question requires three steps:
1. We need to raise the temperature of the ice from 

2. We need to melt the ice.
3. We need to raise the temperature of the water from 

Steps one and three can use the equation:
Step 2 requires the enthalpy of fusion to be multiplied by the mass of the water sample.
Finding these three added heats will give us the total amount of heat needed to raise the temperature from 

AI TutorYour personal study buddy
Question of the DayDaily practice to build your skills
FlashcardsStudy and memorize key concepts
AI SolverStep-by-step problem solutions
Learn by ConceptMaster concepts step by step
Practice TestsTest your skills with exam questions
QuizzesTest your knowledge with a quiz
GamesLearn through free games