Laws of Thermodynamics
Help Questions
Physical Chemistry › Laws of Thermodynamics
A newly discovered element has been determined to have a standard entropy of 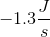
Third Law of Thermodynamics
First Law of Equivalent Exchange
Second Law of Thermodynamics
First Law of Thermodynamics
Zeroth Law of Thermodynamics
Explanation
The Third Law of Thermodynamics states that a pure crystalline substance at absolute zero has an entropy of 
where 
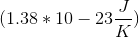
If only one microstate exists for the system, then the entropy is zero 
Remark: keep in mind that entropy changes can be negative, but the entropy of pure compounds cannot be negative.
