Heat Capacity and Specific Heat
Help Questions
Physical Chemistry › Heat Capacity and Specific Heat
What is the specific heat capacity for a 50-gram sample of metal that increases in temperature by 10 degrees celsius when 2000 joules of energy is added?
Explanation
Use the equation:
We can calculate the specific heat capacity for the unknown metal. Since we know the added heat 



A 

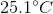


It is impossible to determine
Explanation
Recall that the formula for specific heat is 
Remark: keep in mind that the release of energy and the cooling of the metal will give negative values for both 











