Phases and Properties of Matter - Physical Chemistry
Card 1 of 124
Which of the following forces is considered the weakest?
Which of the following forces is considered the weakest?
Tap to reveal answer
The intermolecular forces from strongest to weakest are ionic, hydrogen bonding, dipole-dipole, then London dispersion forces. All compounds experience some form of London dispersion force, but the force only becomes relevant if no other forces are contributing to the attraction of molecules or atoms.
The intermolecular forces from strongest to weakest are ionic, hydrogen bonding, dipole-dipole, then London dispersion forces. All compounds experience some form of London dispersion force, but the force only becomes relevant if no other forces are contributing to the attraction of molecules or atoms.
← Didn't Know|Knew It →
Which intermolecular force will be the most powerful in a sample of ethanol 

Which intermolecular force will be the most powerful in a sample of ethanol
Tap to reveal answer
Ethanol will not form ions in solution, so we are left with the other three options as plausible answers. Of the three, the strongest force is hydrogen bonding, which only occurs if a hydrogen is directly attached to a nitrogen, oxygen, or fluorine in the molecule. Ethanol has a hydrogen attached to an oxygen, and is thus capable of hydrogen bonding.
Ethanol will not form ions in solution, so we are left with the other three options as plausible answers. Of the three, the strongest force is hydrogen bonding, which only occurs if a hydrogen is directly attached to a nitrogen, oxygen, or fluorine in the molecule. Ethanol has a hydrogen attached to an oxygen, and is thus capable of hydrogen bonding.
← Didn't Know|Knew It →
Which of the following is not an intensive property?
Which of the following is not an intensive property?
Tap to reveal answer
Intensive properties are not dependent on the amount of substance. Melting point, pressure, temperature, and density are some examples of intensive properties. Therefore, volume of a substance is not an example of an intensive property, rather, it is an extensive property which depends on the amount of substance. Some other examples of extensive properties include weight, energy, and electric charge.
Intensive properties are not dependent on the amount of substance. Melting point, pressure, temperature, and density are some examples of intensive properties. Therefore, volume of a substance is not an example of an intensive property, rather, it is an extensive property which depends on the amount of substance. Some other examples of extensive properties include weight, energy, and electric charge.
← Didn't Know|Knew It →
Which of the following is not an intensive property?
Which of the following is not an intensive property?
Tap to reveal answer
Intensive properties are not dependent on the amount of substance. Melting point, pressure, temperature, and density are some examples of intensive properties. Therefore, volume of a substance is not an example of an intensive property, rather, it is an extensive property which depends on the amount of substance. Some other examples of extensive properties include weight, energy, and electric charge.
Intensive properties are not dependent on the amount of substance. Melting point, pressure, temperature, and density are some examples of intensive properties. Therefore, volume of a substance is not an example of an intensive property, rather, it is an extensive property which depends on the amount of substance. Some other examples of extensive properties include weight, energy, and electric charge.
← Didn't Know|Knew It →
Which of the following is not an intensive property?
Which of the following is not an intensive property?
Tap to reveal answer
Intensive properties are not dependent on the amount of substance. Melting point, pressure, temperature, and density are some examples of intensive properties. Therefore, volume of a substance is not an example of an intensive property, rather, it is an extensive property which depends on the amount of substance. Some other examples of extensive properties include weight, energy, and electric charge.
Intensive properties are not dependent on the amount of substance. Melting point, pressure, temperature, and density are some examples of intensive properties. Therefore, volume of a substance is not an example of an intensive property, rather, it is an extensive property which depends on the amount of substance. Some other examples of extensive properties include weight, energy, and electric charge.
← Didn't Know|Knew It →
At constant temperature and moles, which of the following is true regarding pressure and volume of a gas?
At constant temperature and moles, which of the following is true regarding pressure and volume of a gas?
Tap to reveal answer
First, we need to figure out which gas law is applicable here. The question states that temperature and moles are constant. This means that we are dealing with Boyle’s law, which states that pressure is inversely proportional to volume. Inversely proportional means that the pressure decreases as volume increases and vice versa. Note that the relationship is still linear (change in one variable causes a proportional change in the other variable), but the two variables have a negative correlation. Positive correlation means increasing or decreasing a variable would also increase or decrease the other variable, respectively.
First, we need to figure out which gas law is applicable here. The question states that temperature and moles are constant. This means that we are dealing with Boyle’s law, which states that pressure is inversely proportional to volume. Inversely proportional means that the pressure decreases as volume increases and vice versa. Note that the relationship is still linear (change in one variable causes a proportional change in the other variable), but the two variables have a negative correlation. Positive correlation means increasing or decreasing a variable would also increase or decrease the other variable, respectively.
← Didn't Know|Knew It →
According to law, increasing temperature will volume at constant pressure and constant moles.
According to law, increasing temperature will volume at constant pressure and constant moles.
Tap to reveal answer
Recall that Charles’ law defines the relationship between temperature and volume at constant pressure and constant moles. The law states that the two variables are directly proportional to each other. This means that increasing or decreasing temperature will have the same effect on volume. This makes sense because increasing the temperature increases the kinetic energy of the particles. This makes it easier for particles to move away from each other and expand, which results in an increase in volume.
Recall that Charles’ law defines the relationship between temperature and volume at constant pressure and constant moles. The law states that the two variables are directly proportional to each other. This means that increasing or decreasing temperature will have the same effect on volume. This makes sense because increasing the temperature increases the kinetic energy of the particles. This makes it easier for particles to move away from each other and expand, which results in an increase in volume.
← Didn't Know|Knew It →
A sample of argon gas at a pressure of 0.959atm and a temperature of  , occupies a volume of 563mL. If the gas is compressed at constant temperature until its pressure is 1.40atm, what will be the final volume of the sample of gas?
, occupies a volume of 563mL. If the gas is compressed at constant temperature until its pressure is 1.40atm, what will be the final volume of the sample of gas?
A sample of argon gas at a pressure of 0.959atm and a temperature of 
Tap to reveal answer
The argon gas, as the problem states, is under constant temperature with varying volume and pressure, so we need to use Boyle's Law:

We are given the initial pressure, the initial volume, and the final pressure, and need to solve for the final volume. Therefore, we can rearrange Boyle's law to be as follows:

Plug in known values and solve.


The argon gas, as the problem states, is under constant temperature with varying volume and pressure, so we need to use Boyle's Law:
We are given the initial pressure, the initial volume, and the final pressure, and need to solve for the final volume. Therefore, we can rearrange Boyle's law to be as follows:
Plug in known values and solve.
← Didn't Know|Knew It →
A sample of methane gas at a pressure of 0.723atm and a temperature of  , occupies a volume of 16.0L. If the gas is allowed to expand at constant temperature to a volume of 24.2L, what will the pressure of the gas sample be?
, occupies a volume of 16.0L. If the gas is allowed to expand at constant temperature to a volume of 24.2L, what will the pressure of the gas sample be?
A sample of methane gas at a pressure of 0.723atm and a temperature of 
Tap to reveal answer
The methane gas, as the problem states, is under constant temperature with varying volume and pressure, so we need to use Boyle's Law:

We are given the initial pressure, the initial volume, and the final volume and need to solve for the final pressure. Therefore, we can rearrange Boyle's law to be as follows:

Plug in known values and solve.


The methane gas, as the problem states, is under constant temperature with varying volume and pressure, so we need to use Boyle's Law:
We are given the initial pressure, the initial volume, and the final volume and need to solve for the final pressure. Therefore, we can rearrange Boyle's law to be as follows:
Plug in known values and solve.
← Didn't Know|Knew It →
A researcher places a closed piston container at room temperature. He places a Bunsen burner at the bottom of the container and observes that the piston moves up. What can best explain this phenomenon?
A researcher places a closed piston container at room temperature. He places a Bunsen burner at the bottom of the container and observes that the piston moves up. What can best explain this phenomenon?
Tap to reveal answer
There are three main gas laws. Avogadro’s law states that the moles of a gas is directly proportional to the volume (under constant pressure and temperature). Boyle’s law states that the pressure of the gas is inversely proportional to the volume (under constant moles and temperature). This means that the pressure decreases proportionally when the volume increases. Charles’ law states that the temperature is directly proportional to the volume (under constant moles and pressure). The question states that the piston moves up. This means that the volume of the gas is expanding inside and pushing the piston up to make room for the expanding gas. The gas expansion occurs due to increased temperature (Charles’ law).
There are three main gas laws. Avogadro’s law states that the moles of a gas is directly proportional to the volume (under constant pressure and temperature). Boyle’s law states that the pressure of the gas is inversely proportional to the volume (under constant moles and temperature). This means that the pressure decreases proportionally when the volume increases. Charles’ law states that the temperature is directly proportional to the volume (under constant moles and pressure). The question states that the piston moves up. This means that the volume of the gas is expanding inside and pushing the piston up to make room for the expanding gas. The gas expansion occurs due to increased temperature (Charles’ law).
← Didn't Know|Knew It →
An unknown gas is being analyzed in lab. You place  of this gas in a container at
of this gas in a container at  . You observe that the pressure and volume of the gas is
. You observe that the pressure and volume of the gas is  and
and  , respectively. What is the identity of the gas? Assume the gas behaves ideally.
, respectively. What is the identity of the gas? Assume the gas behaves ideally.
An unknown gas is being analyzed in lab. You place 



Tap to reveal answer
To solve this question we need to use the ideal gas law equation:

Above,  is pressure in
is pressure in  ,
,  is volume in liters,
is volume in liters,  is moles,
is moles,  is
is  , and
, and  is temperature in Kelvins. The question gives us pressure, volume, and temperature; therefore, we can solve for
is temperature in Kelvins. The question gives us pressure, volume, and temperature; therefore, we can solve for  . First, we need to convert temperature from Celsius to Kelvins.
. First, we need to convert temperature from Celsius to Kelvins.


Rearrange the ideal gas law and solve for  :
:



The question states that you place one gram of gas in the container. Since we know the moles, we can solve for the molecular weight of the gas and figure out its identity from the molecular weight.

The molecular weight of oxygen gas,  is
is  ; therefore, the unknown gas must be oxygen.
; therefore, the unknown gas must be oxygen.
To solve this question we need to use the ideal gas law equation:
Above, 







Rearrange the ideal gas law and solve for 
The question states that you place one gram of gas in the container. Since we know the moles, we can solve for the molecular weight of the gas and figure out its identity from the molecular weight.
The molecular weight of oxygen gas, 

← Didn't Know|Knew It →
Given the ideal gas law:
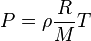
Where  is density,
is density,  is pressure,
is pressure,  is the gas constant,
is the gas constant,  is molar mass, and
is molar mass, and  is temperature.
is temperature.
Based on the Ideal gas law, which of the following are true?
I. Pressure and volume are inversely proportional
II. Pressure and density are inversely proportional
III. Pressure and temperature are directly proportional
IV. Density and temperature are inversely proportional
V. R and M are inversely proportional
Given the ideal gas law:
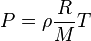
Where 




Based on the Ideal gas law, which of the following are true?
I. Pressure and volume are inversely proportional
II. Pressure and density are inversely proportional
III. Pressure and temperature are directly proportional
IV. Density and temperature are inversely proportional
V. R and M are inversely proportional
Tap to reveal answer
Condition I is true. Pressure and volume are inversely proportional. The ideal gas law with volume can be rederived to show this:
We introduce an expression for moles:
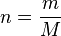

Where  is moles,
is moles,  is mass, and
is mass, and  is molar mass in
is molar mass in 
Rearranging (1) to solve for  and plugging into the following:
and plugging into the following:
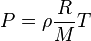
We get:
 (2)
(2)
Finally, we use the following for density, where  is volume, and plug into (2)
is volume, and plug into (2)


The masses cancel out and we have the ideal gas law expressed with volume and moles.
Condition II is false. It is clear from the equation that pressure and density are directly proportional.
Condition III is true because it is clear from the equation that temperature and pressure are directly proportional.
Condition IV is true because density and temperature are on the same side of the equation in the numerator, so the must be inversely proportional.
Condition V is false because the ideal gas constant and molar mass can be rearranged to be on opposite sides of the equation and in the numerator.
Condition I is true. Pressure and volume are inversely proportional. The ideal gas law with volume can be rederived to show this:
We introduce an expression for moles:
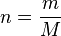
Where 


Rearranging (1) to solve for 
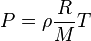
We get:

Finally, we use the following for density, where 
The masses cancel out and we have the ideal gas law expressed with volume and moles.
Condition II is false. It is clear from the equation that pressure and density are directly proportional.
Condition III is true because it is clear from the equation that temperature and pressure are directly proportional.
Condition IV is true because density and temperature are on the same side of the equation in the numerator, so the must be inversely proportional.
Condition V is false because the ideal gas constant and molar mass can be rearranged to be on opposite sides of the equation and in the numerator.
← Didn't Know|Knew It →
Under which conditions do gases deviate from ideality?
I. Low temperature
II. High temperature
III. Low pressure
IV. High pressure
V. Gases always behave ideally
Under which conditions do gases deviate from ideality?
I. Low temperature
II. High temperature
III. Low pressure
IV. High pressure
V. Gases always behave ideally
Tap to reveal answer
Gases deviate from ideal conditions at low temperature and high pressure. This because the postulates of the kinetic molecular theory of gasses ignore the volume of the molecules and all interactions between gas molecules. However, neither are true for real gasses. As the temperature increases, the kinetic energy of the particles can overcome the intermolecular forces of attraction or repulsion between the molecules. At high pressures, and subsequently low volume, the distance between molecules becomes shorter, and therefore intermolecular forces become significant. So, ideal conditions are when the distance between molecules is great, and the energy each molecule has is much greater in relative magnitude to the intermolecular forces. This occurs when the pressure is low, and the temperature is high.
Gases deviate from ideal conditions at low temperature and high pressure. This because the postulates of the kinetic molecular theory of gasses ignore the volume of the molecules and all interactions between gas molecules. However, neither are true for real gasses. As the temperature increases, the kinetic energy of the particles can overcome the intermolecular forces of attraction or repulsion between the molecules. At high pressures, and subsequently low volume, the distance between molecules becomes shorter, and therefore intermolecular forces become significant. So, ideal conditions are when the distance between molecules is great, and the energy each molecule has is much greater in relative magnitude to the intermolecular forces. This occurs when the pressure is low, and the temperature is high.
← Didn't Know|Knew It →
Given the van der Waals Equation:

Which statement accurately identifies and explains the corrections that van der Waals made to the ideal gas law, where the first correction is the  term and the second correction is the
term and the second correction is the  term?
term?
Given the van der Waals Equation:
Which statement accurately identifies and explains the corrections that van der Waals made to the ideal gas law, where the first correction is the 

Tap to reveal answer
All the statements say similar things, but only one correctly identifies the resulting change to volume and pressure. Let's break things down by the correction.
Correction 1:
The correction decreases the volume, since it is subtracting  from the overall volume. As n (or the number of molecules) goes up, volume will go down.
from the overall volume. As n (or the number of molecules) goes up, volume will go down.
Correction 2:
Since we are subtracting  from the overall pressure, we are correcting for molecular attraction by decreasing pressure. We are doing this with a term
from the overall pressure, we are correcting for molecular attraction by decreasing pressure. We are doing this with a term  that gets largerwhen
that gets largerwhen  increases and and gets larger as
increases and and gets larger as  decreases.
decreases.
All the statements say similar things, but only one correctly identifies the resulting change to volume and pressure. Let's break things down by the correction.
Correction 1:
The correction decreases the volume, since it is subtracting 
Correction 2:
Since we are subtracting 



← Didn't Know|Knew It →
When 20 grams of a solute is added to a liter of water, the freezing point becomes  What is the molar mass of the solute?
What is the molar mass of the solute?
Assume that the solute does not make ions in solution.

When 20 grams of a solute is added to a liter of water, the freezing point becomes 
Assume that the solute does not make ions in solution.
Tap to reveal answer
We can use the freezing point depression equation in order to determine the molar mass of the unknown solute.

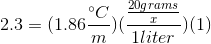

We can use the freezing point depression equation in order to determine the molar mass of the unknown solute.
← Didn't Know|Knew It →
Which of the following is not an intensive property?
Which of the following is not an intensive property?
Tap to reveal answer
Intensive properties are not dependent on the amount of substance. Melting point, pressure, temperature, and density are some examples of intensive properties. Therefore, volume of a substance is not an example of an intensive property, rather, it is an extensive property which depends on the amount of substance. Some other examples of extensive properties include weight, energy, and electric charge.
Intensive properties are not dependent on the amount of substance. Melting point, pressure, temperature, and density are some examples of intensive properties. Therefore, volume of a substance is not an example of an intensive property, rather, it is an extensive property which depends on the amount of substance. Some other examples of extensive properties include weight, energy, and electric charge.
← Didn't Know|Knew It →
A sample of argon gas at a pressure of 0.959atm and a temperature of  , occupies a volume of 563mL. If the gas is compressed at constant temperature until its pressure is 1.40atm, what will be the final volume of the sample of gas?
, occupies a volume of 563mL. If the gas is compressed at constant temperature until its pressure is 1.40atm, what will be the final volume of the sample of gas?
A sample of argon gas at a pressure of 0.959atm and a temperature of 
Tap to reveal answer
The argon gas, as the problem states, is under constant temperature with varying volume and pressure, so we need to use Boyle's Law:

We are given the initial pressure, the initial volume, and the final pressure, and need to solve for the final volume. Therefore, we can rearrange Boyle's law to be as follows:

Plug in known values and solve.


The argon gas, as the problem states, is under constant temperature with varying volume and pressure, so we need to use Boyle's Law:
We are given the initial pressure, the initial volume, and the final pressure, and need to solve for the final volume. Therefore, we can rearrange Boyle's law to be as follows:
Plug in known values and solve.
← Didn't Know|Knew It →
A sample of methane gas at a pressure of 0.723atm and a temperature of  , occupies a volume of 16.0L. If the gas is allowed to expand at constant temperature to a volume of 24.2L, what will the pressure of the gas sample be?
, occupies a volume of 16.0L. If the gas is allowed to expand at constant temperature to a volume of 24.2L, what will the pressure of the gas sample be?
A sample of methane gas at a pressure of 0.723atm and a temperature of 
Tap to reveal answer
The methane gas, as the problem states, is under constant temperature with varying volume and pressure, so we need to use Boyle's Law:

We are given the initial pressure, the initial volume, and the final volume and need to solve for the final pressure. Therefore, we can rearrange Boyle's law to be as follows:

Plug in known values and solve.


The methane gas, as the problem states, is under constant temperature with varying volume and pressure, so we need to use Boyle's Law:
We are given the initial pressure, the initial volume, and the final volume and need to solve for the final pressure. Therefore, we can rearrange Boyle's law to be as follows:
Plug in known values and solve.
← Didn't Know|Knew It →
A researcher places a closed piston container at room temperature. He places a Bunsen burner at the bottom of the container and observes that the piston moves up. What can best explain this phenomenon?
A researcher places a closed piston container at room temperature. He places a Bunsen burner at the bottom of the container and observes that the piston moves up. What can best explain this phenomenon?
Tap to reveal answer
There are three main gas laws. Avogadro’s law states that the moles of a gas is directly proportional to the volume (under constant pressure and temperature). Boyle’s law states that the pressure of the gas is inversely proportional to the volume (under constant moles and temperature). This means that the pressure decreases proportionally when the volume increases. Charles’ law states that the temperature is directly proportional to the volume (under constant moles and pressure). The question states that the piston moves up. This means that the volume of the gas is expanding inside and pushing the piston up to make room for the expanding gas. The gas expansion occurs due to increased temperature (Charles’ law).
There are three main gas laws. Avogadro’s law states that the moles of a gas is directly proportional to the volume (under constant pressure and temperature). Boyle’s law states that the pressure of the gas is inversely proportional to the volume (under constant moles and temperature). This means that the pressure decreases proportionally when the volume increases. Charles’ law states that the temperature is directly proportional to the volume (under constant moles and pressure). The question states that the piston moves up. This means that the volume of the gas is expanding inside and pushing the piston up to make room for the expanding gas. The gas expansion occurs due to increased temperature (Charles’ law).
← Didn't Know|Knew It →
An unknown gas is being analyzed in lab. You place  of this gas in a container at
of this gas in a container at  . You observe that the pressure and volume of the gas is
. You observe that the pressure and volume of the gas is  and
and  , respectively. What is the identity of the gas? Assume the gas behaves ideally.
, respectively. What is the identity of the gas? Assume the gas behaves ideally.
An unknown gas is being analyzed in lab. You place 



Tap to reveal answer
To solve this question we need to use the ideal gas law equation:

Above,  is pressure in
is pressure in  ,
,  is volume in liters,
is volume in liters,  is moles,
is moles,  is
is  , and
, and  is temperature in Kelvins. The question gives us pressure, volume, and temperature; therefore, we can solve for
is temperature in Kelvins. The question gives us pressure, volume, and temperature; therefore, we can solve for  . First, we need to convert temperature from Celsius to Kelvins.
. First, we need to convert temperature from Celsius to Kelvins.


Rearrange the ideal gas law and solve for  :
:



The question states that you place one gram of gas in the container. Since we know the moles, we can solve for the molecular weight of the gas and figure out its identity from the molecular weight.

The molecular weight of oxygen gas,  is
is  ; therefore, the unknown gas must be oxygen.
; therefore, the unknown gas must be oxygen.
To solve this question we need to use the ideal gas law equation:
Above, 







Rearrange the ideal gas law and solve for 
The question states that you place one gram of gas in the container. Since we know the moles, we can solve for the molecular weight of the gas and figure out its identity from the molecular weight.
The molecular weight of oxygen gas, 

← Didn't Know|Knew It →