Nuclear, Quantum, and Molecular Chemistry - Physical Chemistry
Card 1 of 260
What is the hybridization on the nitrogen atom in a molecule of ammonia?
What is the hybridization on the nitrogen atom in a molecule of ammonia?
Tap to reveal answer
The hybridization of an atom can be determined by the number of atoms it is bonded to, as well as the number of lone pairs it has. Two of these variables would be sp, three variables would be sp2, and four would be sp3.
The nitrogen in ammonia is bonded to three atoms of hydrogen, but also has a lone pair in order to satisfy its octet. This means that nitrogen exhibits sp3 hybridization.
The hybridization of an atom can be determined by the number of atoms it is bonded to, as well as the number of lone pairs it has. Two of these variables would be sp, three variables would be sp2, and four would be sp3.
The nitrogen in ammonia is bonded to three atoms of hydrogen, but also has a lone pair in order to satisfy its octet. This means that nitrogen exhibits sp3 hybridization.
← Didn't Know|Knew It →
Which of the following are true regarding  and
and  orbitals?
orbitals?
I. Both  and
and  orbitals can contain a maximum of two electrons
orbitals can contain a maximum of two electrons
II. In a given shell,  orbitals are more numerous because they are spherical in shape
orbitals are more numerous because they are spherical in shape
III.  orbitals have lower energy than
orbitals have lower energy than  orbitals within the same shell
orbitals within the same shell
Which of the following are true regarding 

I. Both 

II. In a given shell, 
III. 

Tap to reveal answer
Orbitals are regions in an electron shell where electrons might be located. There are several types of orbitals such as  , and
, and  . Most elements found on the periodic table contain electrons within one of these orbitals. A characteristic of an orbital is that it can only contain two electrons maximum. A shell might contain multiple orbitals; however, each orbital can only contain two electrons. Each orbital has a unique shape that corresponds to the electron density (the possible location of an electron at a given point in time). The
. Most elements found on the periodic table contain electrons within one of these orbitals. A characteristic of an orbital is that it can only contain two electrons maximum. A shell might contain multiple orbitals; however, each orbital can only contain two electrons. Each orbital has a unique shape that corresponds to the electron density (the possible location of an electron at a given point in time). The  orbital has a spherical shape whereas the
orbital has a spherical shape whereas the  orbital has a dumbbell shape. As mentioned, a shell can contain multiple types of orbitals. A shell can typically contain one
orbital has a dumbbell shape. As mentioned, a shell can contain multiple types of orbitals. A shell can typically contain one  orbital, three
orbital, three  orbitals, five
orbitals, five  orbitals, and seven
orbitals, and seven  orbitals. Remember that the shape of the orbital has no bearing on the amount of orbitals in a shell. An orbital is higher in energy if it is found farther away from the nucleus. The orbitals in order of increasing energy is as follows
orbitals. Remember that the shape of the orbital has no bearing on the amount of orbitals in a shell. An orbital is higher in energy if it is found farther away from the nucleus. The orbitals in order of increasing energy is as follows  . Therefore, an
. Therefore, an  orbital has lower energy than a
orbital has lower energy than a  orbital in the same shell.
orbital in the same shell.
Orbitals are regions in an electron shell where electrons might be located. There are several types of orbitals such as 










← Didn't Know|Knew It →
What is true when comparing the electron configuration of elemental sodium  and sodium cation
and sodium cation  ?
?
What is true when comparing the electron configuration of elemental sodium 

Tap to reveal answer
To answer this question, we need to find the electron configuration of both elemental sodium and sodium cation. If we look at the periodic table we can see that sodium is found on the first column. Since it is found in the first column, sodium has one valence electron. To complete octet, sodium will readily lose an electron and become a positively charged sodium ion. The electron configuration for sodium is  . The electron configuration for sodium ion is
. The electron configuration for sodium ion is  (because it lost its electron in the
(because it lost its electron in the  orbital). This means that elemental sodium has an unpaired electron in its
orbital). This means that elemental sodium has an unpaired electron in its  orbital; the sodium ion has no unpaired electrons. Recall that an unpaired electron can generate its own magnetic field and is called paramagnetic; therefore, solid sodium is paramagnetic. The number of electrons in the
orbital; the sodium ion has no unpaired electrons. Recall that an unpaired electron can generate its own magnetic field and is called paramagnetic; therefore, solid sodium is paramagnetic. The number of electrons in the  orbitals for both sodium and sodium ion is the same (6 electrons total in the
orbitals for both sodium and sodium ion is the same (6 electrons total in the  orbital). The outermost shell of sodium is the third shell (because sodium is located on the third row of periodic table). Elemental sodium contains one electron in the
orbital). The outermost shell of sodium is the third shell (because sodium is located on the third row of periodic table). Elemental sodium contains one electron in the  orbital in the outermost shell whereas the sodium ion contains 6 electrons in its outermost shell.
orbital in the outermost shell whereas the sodium ion contains 6 electrons in its outermost shell.
To answer this question, we need to find the electron configuration of both elemental sodium and sodium cation. If we look at the periodic table we can see that sodium is found on the first column. Since it is found in the first column, sodium has one valence electron. To complete octet, sodium will readily lose an electron and become a positively charged sodium ion. The electron configuration for sodium is 






← Didn't Know|Knew It →
It is observed that a molecule has three hybridized orbitals in its outermost shell. What can you conclude about this molecule?
It is observed that a molecule has three hybridized orbitals in its outermost shell. What can you conclude about this molecule?
Tap to reveal answer
Hybridization is a process involving the fusion, or hybridization, of  and
and  orbitals to form a unique orbital. It is possible for various combinations of
orbitals to form a unique orbital. It is possible for various combinations of  and
and  hybridization. Recall that there is one
hybridization. Recall that there is one  orbital and three
orbital and three  orbitals in each shell. This means that the one
orbitals in each shell. This means that the one  orbital can hybridize with 1, 2, or all 3
orbital can hybridize with 1, 2, or all 3  orbitals. Since there are three total combinations, there are three types of hybridized orbitals. These are
orbitals. Since there are three total combinations, there are three types of hybridized orbitals. These are  ,
,  , and
, and  .
.  orbital has one
orbital has one  and one
and one  orbital hybridized. This means that the
orbital hybridized. This means that the  orbital and the first
orbital and the first  orbital become a new
orbital become a new  orbital. A molecule with
orbital. A molecule with  hybridization will have two
hybridization will have two  orbitals and two
orbitals and two  orbitals. Similarly, an
orbitals. Similarly, an  orbital is made from the hybridization of one
orbital is made from the hybridization of one  and two
and two  orbitals. In
orbitals. In  hybridization, there are three
hybridization, there are three  orbitals and one
orbitals and one  orbital. Finally, an
orbital. Finally, an  orbital has one
orbital has one  and all three
and all three  orbitals; therefore, an
orbitals; therefore, an  hybridized molecule will have four
hybridized molecule will have four  orbitals and no
orbitals and no  orbitals. The question states that there are three hybridized orbitals in this molecule; therefore, this molecule must be
orbitals. The question states that there are three hybridized orbitals in this molecule; therefore, this molecule must be  hybridized. The single
hybridized. The single  orbital is unhybridized because the molecule probably has a double bond. Electrons in
orbital is unhybridized because the molecule probably has a double bond. Electrons in  bonds in double and triple bonds cannot be found in hybridized orbitals; therefore, they need their own
bonds in double and triple bonds cannot be found in hybridized orbitals; therefore, they need their own  orbital. If a molecule has one
orbital. If a molecule has one  bond (double bond), then it will need one
bond (double bond), then it will need one  orbital and will be
orbital and will be  hybridized (because this will give three
hybridized (because this will give three  hybridized orbitals and one
hybridized orbitals and one  orbital). If it has two
orbital). If it has two  bonds (triple bond), then it will need two
bonds (triple bond), then it will need two  orbitals and will be
orbitals and will be  hybridized. If a molecule has all
hybridized. If a molecule has all  bonds (single bonds), then the molecule will require no empty
bonds (single bonds), then the molecule will require no empty  orbitals for the delocalized electrons, and will be
orbitals for the delocalized electrons, and will be  hybridized.
hybridized.
Hybridization is a process involving the fusion, or hybridization, of 














































← Didn't Know|Knew It →
Which of the following is true regarding carbon tetrachloride?
Which of the following is true regarding carbon tetrachloride?
Tap to reveal answer
Carbon tetrachloride,  , has a central carbon atom attached to four chlorine atoms. The bonds between the carbon atom and chlorine atoms are single covalent bonds. The electrons in a single bond (
, has a central carbon atom attached to four chlorine atoms. The bonds between the carbon atom and chlorine atoms are single covalent bonds. The electrons in a single bond ( bond) can be found in hybridized orbitals. Since carbon tetrachloride only has single bonds, the carbon atom can hybridize all of its orbitals (one
bond) can be found in hybridized orbitals. Since carbon tetrachloride only has single bonds, the carbon atom can hybridize all of its orbitals (one  and three
and three  ) in the outermost shell and form a
) in the outermost shell and form a  hybridization; therefore, three
hybridization; therefore, three  orbitals and one
orbitals and one  orbital participate in hybridization leading us to the correct answer. Carbon dioxide,
orbital participate in hybridization leading us to the correct answer. Carbon dioxide,  , has a central carbon atom bonded to two oxygen atoms. To complete octet, carbon and oxygen atoms have double bonds. This means that carbon dioxide has two
, has a central carbon atom bonded to two oxygen atoms. To complete octet, carbon and oxygen atoms have double bonds. This means that carbon dioxide has two  bonds (two double bonds). Recall that electrons in
bonds (two double bonds). Recall that electrons in  bonds cannot reside in hybridized orbitals; therefore, to accommodate the two
bonds cannot reside in hybridized orbitals; therefore, to accommodate the two  bonds we need two empty, unhybridized
bonds we need two empty, unhybridized  orbitals. This means that carbon dioxide will have hybridization of one
orbitals. This means that carbon dioxide will have hybridization of one  and one
and one  orbital, giving it an
orbital, giving it an  hybridization.
hybridization.
Carbon tetrachloride, 














← Didn't Know|Knew It →
Which of the following quantities is zero for photons?
Which of the following quantities is zero for photons?
Tap to reveal answer
Photons are fundamental particles that make up electromagnetic waves (light). The key aspect of photon is that it has very high speed  and no mass; therefore, mass for photons is always zero.
and no mass; therefore, mass for photons is always zero.
Photons are fundamental particles that make up electromagnetic waves (light). The key aspect of photon is that it has very high speed 
← Didn't Know|Knew It →
Photons in ultraviolet rays have a wavelength of 10nm. What is the energy of photons in radio waves?
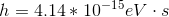
Photons in ultraviolet rays have a wavelength of 10nm. What is the energy of photons in radio waves?
Tap to reveal answer
To solve this question, we need to use the equation relating energy of a wave to its wavelength.

Here,  is the energy,
is the energy,  is Planck’s constant,
is Planck’s constant,  is speed of light (for us it’s always
is speed of light (for us it’s always  ), and
), and  is the wavelength (in meters). The energy of the ultraviolet rays is
is the wavelength (in meters). The energy of the ultraviolet rays is


If we look at the electromagnetic spectrum we will notice that the wavelength of radio waves is a lot higher than ultraviolet rays. Since energy is inversely proportional to wavelength we can conclude that the energy of radio waves is lower than ultraviolet's. The only answer choice with an energy lower than ultraviolet rays energy is 1.24neV.
To solve this question, we need to use the equation relating energy of a wave to its wavelength.
Here, 




If we look at the electromagnetic spectrum we will notice that the wavelength of radio waves is a lot higher than ultraviolet rays. Since energy is inversely proportional to wavelength we can conclude that the energy of radio waves is lower than ultraviolet's. The only answer choice with an energy lower than ultraviolet rays energy is 1.24neV.
← Didn't Know|Knew It →
Which of the following is/are the same for photons in gamma rays and in visible light?
I. frequency
II. wavelength
III. speed
Which of the following is/are the same for photons in gamma rays and in visible light?
I. frequency
II. wavelength
III. speed
Tap to reveal answer
Electromagnetic spectrum is a spectrum of different types of light. Each type of electromagnetic wave differs from one another based on the frequency and wavelength; however, the speed of every electromagnetic wave in air is  . The wave equation relates the speed of an electromagnetic to its wavelength and frequency as follows.
. The wave equation relates the speed of an electromagnetic to its wavelength and frequency as follows.

Here,  is speed of light,
is speed of light,  is wavelength, and
is wavelength, and  is frequency. Since
is frequency. Since  is always constant, wavelength and frequency are inversely proportional to each other. This means that if the wavelength increases the frequency decreases.
is always constant, wavelength and frequency are inversely proportional to each other. This means that if the wavelength increases the frequency decreases.
Electromagnetic spectrum is a spectrum of different types of light. Each type of electromagnetic wave differs from one another based on the frequency and wavelength; however, the speed of every electromagnetic wave in air is 
Here, 



← Didn't Know|Knew It →
Which of the following sets of quantum numbers ( ) could correspond to a
) could correspond to a  orbital?
orbital?
Which of the following sets of quantum numbers (

Tap to reveal answer
Since we're looking at the  orbital, we know
orbital, we know  . The range of possible values for
. The range of possible values for  is 0 to
is 0 to  . Possible values for
. Possible values for  range
range  to
to  . Therefore, among the answer choices,
. Therefore, among the answer choices,  is the only possible combination of quantum numbers corresponding to an
is the only possible combination of quantum numbers corresponding to an  orbital.
orbital.
Since we're looking at the 








← Didn't Know|Knew It →
Which of the following sets of quantum numbers could correspond to a  orbital?
orbital?
Which of the following sets of quantum numbers could correspond to a 
Tap to reveal answer
Since we're looking at the  orbital, we know
orbital, we know  . The range of possible values for
. The range of possible values for  is 0 to
is 0 to  . Possible values for
. Possible values for  range
range  to
to  . Therefore, among the answer choices,
. Therefore, among the answer choices,



is the only possible combination of quantum numbers corresponding to a  orbital.
orbital.
Since we're looking at the 






is the only possible combination of quantum numbers corresponding to a 
← Didn't Know|Knew It →
How many subshells are there with n = 4 in an atom?
How many subshells are there with n = 4 in an atom?
Tap to reveal answer
The types of subshells, from smallest to largest, are as follows: s, p, d, and f. These four subshells correspond respectively to the following quantum numbers: 0, 1, 2, and 3. The total number of sublevels with n = 4 is n or 4: 4s, 4p, 4d and 4f.
The types of subshells, from smallest to largest, are as follows: s, p, d, and f. These four subshells correspond respectively to the following quantum numbers: 0, 1, 2, and 3. The total number of sublevels with n = 4 is n or 4: 4s, 4p, 4d and 4f.
← Didn't Know|Knew It →
Which of the following is/are true regarding the principal quantum number?
I. Principal quantum number signifies the energy level
II. Principal quantum number can never equal zero
III. Principal quantum number can be positive or negative
Which of the following is/are true regarding the principal quantum number?
I. Principal quantum number signifies the energy level
II. Principal quantum number can never equal zero
III. Principal quantum number can be positive or negative
Tap to reveal answer
Quantum numbers are fancy coordinate systems that describe the potential location of an electron within an atom. The first quantum number is called the principal quantum number and it signifies the shell the electron is located in. Recall that electron shells are discrete orbits in an atom that have discrete energy; therefore, the principal quantum number signifies the energy level of an electron.
The principal quantum number is always an integer and is always greater than zero. If  the electron is found within the first shell, if
the electron is found within the first shell, if  then the electron is found within the second shell, and so and and so forth. Also, since it is always greater than zero, the principal quantum number can never be negative.
then the electron is found within the second shell, and so and and so forth. Also, since it is always greater than zero, the principal quantum number can never be negative.
Quantum numbers are fancy coordinate systems that describe the potential location of an electron within an atom. The first quantum number is called the principal quantum number and it signifies the shell the electron is located in. Recall that electron shells are discrete orbits in an atom that have discrete energy; therefore, the principal quantum number signifies the energy level of an electron.
The principal quantum number is always an integer and is always greater than zero. If 

← Didn't Know|Knew It →
What can be concluded about the quantum numbers for potassium and potassium ion?
What can be concluded about the quantum numbers for potassium and potassium ion?
Tap to reveal answer
Potassium has one valence electron. This means that there is one electron in its outermost shell (4th shell). Potassium ion, on the other hand, loses an electron and has a complete octet (has eight valence electrons) in its 3rd shell. Recall that the principal quantum number signifies the shell. Since the valence electron of potassium is found in the fourth shell,  . Similarly, the valence electrons of potassium ion are found in the third shell and
. Similarly, the valence electrons of potassium ion are found in the third shell and  for them. Valence electron of potassium has the higher principal quantum number.
for them. Valence electron of potassium has the higher principal quantum number.
Orbital angular momentum number ( ) is the second quantum number and it signifies the type of orbital. It is always greater than or equal to zero. There are four main types of orbital: s, p, d, and f. Each orbital can hold two electrons. In a given shell, there are one ‘s’ orbital, three ‘p’ orbitals, five ‘d’ orbitals, and seven ‘f’ orbitals. ‘l’ = 0 for ‘s’ orbitals, ‘l’ = 1 for ‘p’ orbitals, ‘l’ = 2 for ‘d’ orbitals, and ‘l’ = 3 for ‘f’ orbitals. In potassium, there is only one valence electron; therefore, there is only one electron in the fourth shell and it can fit into the ‘s’ orbital. In potassium ion, there are eight valence electrons; therefore, two electrons can be found in the ‘s’ orbital and the remaining six electrons can be found in the three ‘p’ orbitals. Not all valence electrons of potassium ion and potassium have different ‘l’ value. This is because valence electron of potassium and two of the valence electrons of potassium ion are found in the ‘s’ orbital (
) is the second quantum number and it signifies the type of orbital. It is always greater than or equal to zero. There are four main types of orbital: s, p, d, and f. Each orbital can hold two electrons. In a given shell, there are one ‘s’ orbital, three ‘p’ orbitals, five ‘d’ orbitals, and seven ‘f’ orbitals. ‘l’ = 0 for ‘s’ orbitals, ‘l’ = 1 for ‘p’ orbitals, ‘l’ = 2 for ‘d’ orbitals, and ‘l’ = 3 for ‘f’ orbitals. In potassium, there is only one valence electron; therefore, there is only one electron in the fourth shell and it can fit into the ‘s’ orbital. In potassium ion, there are eight valence electrons; therefore, two electrons can be found in the ‘s’ orbital and the remaining six electrons can be found in the three ‘p’ orbitals. Not all valence electrons of potassium ion and potassium have different ‘l’ value. This is because valence electron of potassium and two of the valence electrons of potassium ion are found in the ‘s’ orbital ( ).
).
Electrons found in higher shell numbers have higher energy. Valence electron of potassium is found in the fourth shell; therefore, it will have a higher energy than any of the valence electrons of potassium ion.
Potassium has one valence electron. This means that there is one electron in its outermost shell (4th shell). Potassium ion, on the other hand, loses an electron and has a complete octet (has eight valence electrons) in its 3rd shell. Recall that the principal quantum number signifies the shell. Since the valence electron of potassium is found in the fourth shell, 

Orbital angular momentum number (

Electrons found in higher shell numbers have higher energy. Valence electron of potassium is found in the fourth shell; therefore, it will have a higher energy than any of the valence electrons of potassium ion.
← Didn't Know|Knew It →
Consider the following descriptions of quantum numbers:
A: energy level within a subshell
B: shape of orbital
C: spin of electron
D: energy level
Which of the following is the correct pairing of quantum numbers (1st, 2nd, 3rd, and 4th) with the given descriptions?
Consider the following descriptions of quantum numbers:
A: energy level within a subshell
B: shape of orbital
C: spin of electron
D: energy level
Which of the following is the correct pairing of quantum numbers (1st, 2nd, 3rd, and 4th) with the given descriptions?
Tap to reveal answer
There are four quantum numbers. The first quantum, or principal quantum number, is designated by the letter ‘n’. It signifies the electron shell, or the energy level of the electron. The second quantum number, or orbital angular momentum quantum number, is designated by the letter ‘l’. It signifies the shape (or type) of the orbital. The third quantum number, or magnetic quantum number, is designated by  . This signifies the energy level within a subshell. Each orbital can be located in different orientations in space. For example, each of the three ‘p’ orbitals are oriented differently in space and have different energy levels. The third quantum number describes this phenomenon. Finally, the fourth quantum number, or spin quantum number, is designated by
. This signifies the energy level within a subshell. Each orbital can be located in different orientations in space. For example, each of the three ‘p’ orbitals are oriented differently in space and have different energy levels. The third quantum number describes this phenomenon. Finally, the fourth quantum number, or spin quantum number, is designated by  and describes the spin of the electron. An electron can spin clockwise or counterclockwise.
and describes the spin of the electron. An electron can spin clockwise or counterclockwise.  describes the direction of the spin. Since an electron can only rotate two ways,
describes the direction of the spin. Since an electron can only rotate two ways,  can only be two values
can only be two values  or
or  .
.
These four numbers together describe the potential location of an electron inside an atom. Note that no two electrons can have the same set of quantum numbers (meaning at least one of the four numbers will be different).
There are four quantum numbers. The first quantum, or principal quantum number, is designated by the letter ‘n’. It signifies the electron shell, or the energy level of the electron. The second quantum number, or orbital angular momentum quantum number, is designated by the letter ‘l’. It signifies the shape (or type) of the orbital. The third quantum number, or magnetic quantum number, is designated by 





These four numbers together describe the potential location of an electron inside an atom. Note that no two electrons can have the same set of quantum numbers (meaning at least one of the four numbers will be different).
← Didn't Know|Knew It →
Which of the following is true regarding ethene? (The electrons in answer choices refer to carbon electrons)
Which of the following is true regarding ethene? (The electrons in answer choices refer to carbon electrons)
Tap to reveal answer
Ethene, or  , has a carbon-carbon single (
, has a carbon-carbon single ( ) and double (
) and double ( ) bond. Recall that a
) bond. Recall that a  bond can be found in hybridized orbitals whereas a
bond can be found in hybridized orbitals whereas a  bond cannot. This means that the carbon atoms in ethene hybridize the single ‘s’ orbital and two of the ‘p’ orbitals, forming a
bond cannot. This means that the carbon atoms in ethene hybridize the single ‘s’ orbital and two of the ‘p’ orbitals, forming a  hybridization. The
hybridization. The  bonds (
bonds ( ) are found in these three hybridized orbitals. The remaining unhybridized ‘p’ orbital will house the two electrons in the
) are found in these three hybridized orbitals. The remaining unhybridized ‘p’ orbital will house the two electrons in the  bond.
bond.
The orbital angular momentum number is the second quantum number and it signifies the type of orbital. An electron found in a ‘p’ orbital will always have an  . Since both electrons in the
. Since both electrons in the  bond are found in the ‘p’ orbital, the ‘l’ value for both electrons is the same.
bond are found in the ‘p’ orbital, the ‘l’ value for both electrons is the same.
The principal quantum number is the first quantum number and it signifies the shell or energy level of an electron. All electrons involved in bonds are found in carbon’s outermost shell (2nd shell); therefore, they will all have an  . Remember that no two electrons can have the same set of quantum numbers (regardless of whether the electrons are found as lone pairs, in
. Remember that no two electrons can have the same set of quantum numbers (regardless of whether the electrons are found as lone pairs, in  bonds, or in
bonds, or in  bonds).
bonds).
Ethene, or 








The orbital angular momentum number is the second quantum number and it signifies the type of orbital. An electron found in a ‘p’ orbital will always have an 

The principal quantum number is the first quantum number and it signifies the shell or energy level of an electron. All electrons involved in bonds are found in carbon’s outermost shell (2nd shell); therefore, they will all have an 


← Didn't Know|Knew It →
Which of the following set of quantum numbers is not valid?
Which of the following set of quantum numbers is not valid?
Tap to reveal answer
The principle quantum number (n) and the angular quantum number (l) are acceptable. However, the magnetic quantum number (m) is restricted to lie between -l and l. Therefore, for l=2, the only possible numbers for m are -2, -1, 0, 1, 2.
The principle quantum number (n) and the angular quantum number (l) are acceptable. However, the magnetic quantum number (m) is restricted to lie between -l and l. Therefore, for l=2, the only possible numbers for m are -2, -1, 0, 1, 2.
← Didn't Know|Knew It →
After undergoing emission a molecule is stable and after undergoing absorption a molecule is stable.
After undergoing emission a molecule is stable and after undergoing absorption a molecule is stable.
Tap to reveal answer
Emission is the process of releasing (or emitting) photons from an atom whereas absorption is the process of absorbing photons. After emission, the atom releases energy (in the form of photons) and goes to a lower energy state. In absorption, however, the atom absorbs energy and goes to a higher energy state. Recall that lower energy always means more stable; therefore, after emission, an atom or molecule goes to a more stable state whereas after absorption it goes to a less stable state.
Emission is the process of releasing (or emitting) photons from an atom whereas absorption is the process of absorbing photons. After emission, the atom releases energy (in the form of photons) and goes to a lower energy state. In absorption, however, the atom absorbs energy and goes to a higher energy state. Recall that lower energy always means more stable; therefore, after emission, an atom or molecule goes to a more stable state whereas after absorption it goes to a less stable state.
← Didn't Know|Knew It →
Which statement best characterizes a covalent bond?
Which statement best characterizes a covalent bond?
Tap to reveal answer
To achieve an octet of valence electrons, atoms can share electrons so that all atoms participating in the bond will have full valence shells. Covalent bonds, by definition, result from the sharing of one or more pairs of valence electrons.
To achieve an octet of valence electrons, atoms can share electrons so that all atoms participating in the bond will have full valence shells. Covalent bonds, by definition, result from the sharing of one or more pairs of valence electrons.
← Didn't Know|Knew It →
How many single covalent bonds would the element sulfur be expected to form in order to obey the octet rule?
How many single covalent bonds would the element sulfur be expected to form in order to obey the octet rule?
Tap to reveal answer
The key to this problem is that electrons in covalent bonds are shared and therefore "belong" to both of the bonded atoms. Sulfur is a nonmetal in group 6A , and therefore has 6 valence electrons. In order to obey the octet rule, it needs to gain 2 electrons . It can do this by forming 2 single covalent bonds.
The key to this problem is that electrons in covalent bonds are shared and therefore "belong" to both of the bonded atoms. Sulfur is a nonmetal in group 6A , and therefore has 6 valence electrons. In order to obey the octet rule, it needs to gain 2 electrons . It can do this by forming 2 single covalent bonds.
← Didn't Know|Knew It →
How many single covalent bonds would the element selenium be expected to form in order to obey the octet rule?
How many single covalent bonds would the element selenium be expected to form in order to obey the octet rule?
Tap to reveal answer
The key to this problem is that electrons in covalent bonds are shared and therefore "belong" to both of the bonded atoms. Selenium is a nonmetal in group 6A , and therefore has 6 valence electrons. In order to obey the octet rule, it needs to gain 2 electrons. It can do this by forming 2 single covalent bonds.
The key to this problem is that electrons in covalent bonds are shared and therefore "belong" to both of the bonded atoms. Selenium is a nonmetal in group 6A , and therefore has 6 valence electrons. In order to obey the octet rule, it needs to gain 2 electrons. It can do this by forming 2 single covalent bonds.
← Didn't Know|Knew It →