Heat Capacity and Specific Heat - Physical Chemistry
Card 1 of 8
What is the specific heat capacity for a 50-gram sample of metal that increases in temperature by 10 degrees celsius when 2000 joules of energy is added?
What is the specific heat capacity for a 50-gram sample of metal that increases in temperature by 10 degrees celsius when 2000 joules of energy is added?
Tap to reveal answer
Use the equation:

We can calculate the specific heat capacity for the unknown metal. Since we know the added heat  , the mass of the sample
, the mass of the sample  , and the change in temperature
, and the change in temperature  , we can solve for
, we can solve for  .
.

Use the equation:
We can calculate the specific heat capacity for the unknown metal. Since we know the added heat 



← Didn't Know|Knew It →
A  sample of an unknown metal at
sample of an unknown metal at  is immersed in water and is allowed to reach equilibrium. If final temperature of the system is
is immersed in water and is allowed to reach equilibrium. If final temperature of the system is 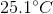 and
and  of energy are released, what is the identity of the metal?
of energy are released, what is the identity of the metal?

A 

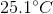


Tap to reveal answer
Recall that the formula for specific heat is  . Rearranging the equation for specific heat (c) yields
. Rearranging the equation for specific heat (c) yields 
Remark: keep in mind that the release of energy and the cooling of the metal will give negative values for both  and
and  .
.
Recall that the formula for specific heat is 
Remark: keep in mind that the release of energy and the cooling of the metal will give negative values for both 

← Didn't Know|Knew It →
What is the specific heat capacity for a 50-gram sample of metal that increases in temperature by 10 degrees celsius when 2000 joules of energy is added?
What is the specific heat capacity for a 50-gram sample of metal that increases in temperature by 10 degrees celsius when 2000 joules of energy is added?
Tap to reveal answer
Use the equation:

We can calculate the specific heat capacity for the unknown metal. Since we know the added heat  , the mass of the sample
, the mass of the sample  , and the change in temperature
, and the change in temperature  , we can solve for
, we can solve for  .
.

Use the equation:
We can calculate the specific heat capacity for the unknown metal. Since we know the added heat 



← Didn't Know|Knew It →
A  sample of an unknown metal at
sample of an unknown metal at  is immersed in water and is allowed to reach equilibrium. If final temperature of the system is
is immersed in water and is allowed to reach equilibrium. If final temperature of the system is 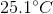 and
and  of energy are released, what is the identity of the metal?
of energy are released, what is the identity of the metal?

A 

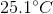


Tap to reveal answer
Recall that the formula for specific heat is  . Rearranging the equation for specific heat (c) yields
. Rearranging the equation for specific heat (c) yields 
Remark: keep in mind that the release of energy and the cooling of the metal will give negative values for both  and
and  .
.
Recall that the formula for specific heat is 
Remark: keep in mind that the release of energy and the cooling of the metal will give negative values for both 

← Didn't Know|Knew It →
What is the specific heat capacity for a 50-gram sample of metal that increases in temperature by 10 degrees celsius when 2000 joules of energy is added?
What is the specific heat capacity for a 50-gram sample of metal that increases in temperature by 10 degrees celsius when 2000 joules of energy is added?
Tap to reveal answer
Use the equation:

We can calculate the specific heat capacity for the unknown metal. Since we know the added heat  , the mass of the sample
, the mass of the sample  , and the change in temperature
, and the change in temperature  , we can solve for
, we can solve for  .
.

Use the equation:
We can calculate the specific heat capacity for the unknown metal. Since we know the added heat 



← Didn't Know|Knew It →
A  sample of an unknown metal at
sample of an unknown metal at  is immersed in water and is allowed to reach equilibrium. If final temperature of the system is
is immersed in water and is allowed to reach equilibrium. If final temperature of the system is 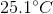 and
and  of energy are released, what is the identity of the metal?
of energy are released, what is the identity of the metal?

A 



Tap to reveal answer
Recall that the formula for specific heat is  . Rearranging the equation for specific heat (c) yields
. Rearranging the equation for specific heat (c) yields 
Remark: keep in mind that the release of energy and the cooling of the metal will give negative values for both  and
and  .
.
Recall that the formula for specific heat is 
Remark: keep in mind that the release of energy and the cooling of the metal will give negative values for both 

← Didn't Know|Knew It →
What is the specific heat capacity for a 50-gram sample of metal that increases in temperature by 10 degrees celsius when 2000 joules of energy is added?
What is the specific heat capacity for a 50-gram sample of metal that increases in temperature by 10 degrees celsius when 2000 joules of energy is added?
Tap to reveal answer
Use the equation:

We can calculate the specific heat capacity for the unknown metal. Since we know the added heat  , the mass of the sample
, the mass of the sample  , and the change in temperature
, and the change in temperature  , we can solve for
, we can solve for  .
.

Use the equation:
We can calculate the specific heat capacity for the unknown metal. Since we know the added heat 



← Didn't Know|Knew It →
A  sample of an unknown metal at
sample of an unknown metal at  is immersed in water and is allowed to reach equilibrium. If final temperature of the system is
is immersed in water and is allowed to reach equilibrium. If final temperature of the system is 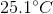 and
and  of energy are released, what is the identity of the metal?
of energy are released, what is the identity of the metal?

A 



Tap to reveal answer
Recall that the formula for specific heat is  . Rearranging the equation for specific heat (c) yields
. Rearranging the equation for specific heat (c) yields 
Remark: keep in mind that the release of energy and the cooling of the metal will give negative values for both  and
and  .
.
Recall that the formula for specific heat is 
Remark: keep in mind that the release of energy and the cooling of the metal will give negative values for both 

← Didn't Know|Knew It →