Gibbs Free Energy - Physical Chemistry
Card 1 of 16
For Constant Temperature, Gibbs Free Energy is defined as:

Where ,  is the change in Gibbs Free Energy,
is the change in Gibbs Free Energy,  is the change in enthalpy,
is the change in enthalpy,  is temperature, and
is temperature, and  is the change in entropy.
is the change in entropy.
Which of the following scenarios is not possible?
For Constant Temperature, Gibbs Free Energy is defined as:
Where , 



Which of the following scenarios is not possible?
Tap to reveal answer
The following condition is not possible:

This is because if enthalpy is positive, and entropy is negative, the negative sign in front of the temperature term in the formula becomes positive. Addition of 2 positive numbers can not be negative. Plugging in arbitrary numbers into the other conditions can show they are all possible.
Take the following condition:

Then Gibbs free energy  can either be positive or negative, depending on the magnitude of enthalpy, entropy, and temperature. (If enthalpy is much larger than entropy and temperature, then the difference will be positive, but if entropy *
can either be positive or negative, depending on the magnitude of enthalpy, entropy, and temperature. (If enthalpy is much larger than entropy and temperature, then the difference will be positive, but if entropy *  is greater than the enthalpy, then the difference will be negative).
is greater than the enthalpy, then the difference will be negative).
The following condition is not possible:
This is because if enthalpy is positive, and entropy is negative, the negative sign in front of the temperature term in the formula becomes positive. Addition of 2 positive numbers can not be negative. Plugging in arbitrary numbers into the other conditions can show they are all possible.
Take the following condition:
Then Gibbs free energy 

← Didn't Know|Knew It →
For constant temperature, Gibbs free energy is defined as:

Where ,  is the change in Gibbs free energy,
is the change in Gibbs free energy,  is the change in enthalpy,
is the change in enthalpy,  is temperature, and
is temperature, and 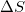 is the change in entropy.
is the change in entropy.
Given that a system is spontaneous, which of the following states are possible?
I. 
II. 
III. 
IV. 
For constant temperature, Gibbs free energy is defined as:
Where , 


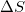
Given that a system is spontaneous, which of the following states are possible?
I.
II.
III.
IV.
Tap to reveal answer
Condition I is always true. Condition II is never true, as Gibbs free energy cannot be negative if enthalpy is positive and entropy is negative. Condition III may be true if temperature is very high (this is the scenario when the  term dominates the
term dominates the  term. Condition IV is not possible because
term. Condition IV is not possible because  and we were given a system with a Gibbs free Energy that is
and we were given a system with a Gibbs free Energy that is  (we were told the system was spontaneous).
(we were told the system was spontaneous).
Condition I is always true. Condition II is never true, as Gibbs free energy cannot be negative if enthalpy is positive and entropy is negative. Condition III may be true if temperature is very high (this is the scenario when the 



← Didn't Know|Knew It →
The enthalpy of a reaction is  and the entropy of a reaction is
and the entropy of a reaction is  . Which of the following is the Gibbs free energy (in
. Which of the following is the Gibbs free energy (in  ) of this reaction?
) of this reaction?
The enthalpy of a reaction is 


Tap to reveal answer
Gibbs free energy of a system can be solved using the following equation.

where  is change in Gibbs free energy,
is change in Gibbs free energy,  is change in enthalpy,
is change in enthalpy,  is temperature in Kelvins and
is temperature in Kelvins and  is change in entropy. To solve for
is change in entropy. To solve for  we need all three of the variables. We are not given the temperature; therefore, we cannot solve for Gibbs free energy.
we need all three of the variables. We are not given the temperature; therefore, we cannot solve for Gibbs free energy.
Gibbs free energy of a system can be solved using the following equation.
where 




← Didn't Know|Knew It →
In an exergonic reaction, products will have Gibbs free energy and the reaction is .
In an exergonic reaction, products will have Gibbs free energy and the reaction is .
Tap to reveal answer
Exergonic reaction suggests that the Gibbs free energy is negative. Since the change in Gibbs free energy is defined as Gibbs free energy of products - Gibbs free energy of reactants, a negative change in Gibbs free energy suggests that the products have a lower Gibbs free energy than reactants. A reaction is spontaneous if it has negative Gibbs free energy; therefore, exergonic reactions are always spontaneous. This is because the reaction is producing a more stable product (lower energy) from a less stable reactant (higher energy).
Exergonic reaction suggests that the Gibbs free energy is negative. Since the change in Gibbs free energy is defined as Gibbs free energy of products - Gibbs free energy of reactants, a negative change in Gibbs free energy suggests that the products have a lower Gibbs free energy than reactants. A reaction is spontaneous if it has negative Gibbs free energy; therefore, exergonic reactions are always spontaneous. This is because the reaction is producing a more stable product (lower energy) from a less stable reactant (higher energy).
← Didn't Know|Knew It →
In an exergonic reaction, products will have Gibbs free energy and the reaction is .
In an exergonic reaction, products will have Gibbs free energy and the reaction is .
Tap to reveal answer
Exergonic reaction suggests that the Gibbs free energy is negative. Since the change in Gibbs free energy is defined as Gibbs free energy of products - Gibbs free energy of reactants, a negative change in Gibbs free energy suggests that the products have a lower Gibbs free energy than reactants. A reaction is spontaneous if it has negative Gibbs free energy; therefore, exergonic reactions are always spontaneous. This is because the reaction is producing a more stable product (lower energy) from a less stable reactant (higher energy).
Exergonic reaction suggests that the Gibbs free energy is negative. Since the change in Gibbs free energy is defined as Gibbs free energy of products - Gibbs free energy of reactants, a negative change in Gibbs free energy suggests that the products have a lower Gibbs free energy than reactants. A reaction is spontaneous if it has negative Gibbs free energy; therefore, exergonic reactions are always spontaneous. This is because the reaction is producing a more stable product (lower energy) from a less stable reactant (higher energy).
← Didn't Know|Knew It →
For Constant Temperature, Gibbs Free Energy is defined as:

Where ,  is the change in Gibbs Free Energy,
is the change in Gibbs Free Energy,  is the change in enthalpy,
is the change in enthalpy,  is temperature, and
is temperature, and  is the change in entropy.
is the change in entropy.
Which of the following scenarios is not possible?
For Constant Temperature, Gibbs Free Energy is defined as:
Where , 



Which of the following scenarios is not possible?
Tap to reveal answer
The following condition is not possible:

This is because if enthalpy is positive, and entropy is negative, the negative sign in front of the temperature term in the formula becomes positive. Addition of 2 positive numbers can not be negative. Plugging in arbitrary numbers into the other conditions can show they are all possible.
Take the following condition:

Then Gibbs free energy  can either be positive or negative, depending on the magnitude of enthalpy, entropy, and temperature. (If enthalpy is much larger than entropy and temperature, then the difference will be positive, but if entropy *
can either be positive or negative, depending on the magnitude of enthalpy, entropy, and temperature. (If enthalpy is much larger than entropy and temperature, then the difference will be positive, but if entropy *  is greater than the enthalpy, then the difference will be negative).
is greater than the enthalpy, then the difference will be negative).
The following condition is not possible:
This is because if enthalpy is positive, and entropy is negative, the negative sign in front of the temperature term in the formula becomes positive. Addition of 2 positive numbers can not be negative. Plugging in arbitrary numbers into the other conditions can show they are all possible.
Take the following condition:
Then Gibbs free energy 

← Didn't Know|Knew It →
For constant temperature, Gibbs free energy is defined as:

Where ,  is the change in Gibbs free energy,
is the change in Gibbs free energy,  is the change in enthalpy,
is the change in enthalpy,  is temperature, and
is temperature, and 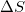 is the change in entropy.
is the change in entropy.
Given that a system is spontaneous, which of the following states are possible?
I. 
II. 
III. 
IV. 
For constant temperature, Gibbs free energy is defined as:
Where , 


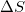
Given that a system is spontaneous, which of the following states are possible?
I.
II.
III.
IV.
Tap to reveal answer
Condition I is always true. Condition II is never true, as Gibbs free energy cannot be negative if enthalpy is positive and entropy is negative. Condition III may be true if temperature is very high (this is the scenario when the  term dominates the
term dominates the  term. Condition IV is not possible because
term. Condition IV is not possible because  and we were given a system with a Gibbs free Energy that is
and we were given a system with a Gibbs free Energy that is  (we were told the system was spontaneous).
(we were told the system was spontaneous).
Condition I is always true. Condition II is never true, as Gibbs free energy cannot be negative if enthalpy is positive and entropy is negative. Condition III may be true if temperature is very high (this is the scenario when the 



← Didn't Know|Knew It →
The enthalpy of a reaction is  and the entropy of a reaction is
and the entropy of a reaction is  . Which of the following is the Gibbs free energy (in
. Which of the following is the Gibbs free energy (in  ) of this reaction?
) of this reaction?
The enthalpy of a reaction is 


Tap to reveal answer
Gibbs free energy of a system can be solved using the following equation.

where  is change in Gibbs free energy,
is change in Gibbs free energy,  is change in enthalpy,
is change in enthalpy,  is temperature in Kelvins and
is temperature in Kelvins and  is change in entropy. To solve for
is change in entropy. To solve for  we need all three of the variables. We are not given the temperature; therefore, we cannot solve for Gibbs free energy.
we need all three of the variables. We are not given the temperature; therefore, we cannot solve for Gibbs free energy.
Gibbs free energy of a system can be solved using the following equation.
where 




← Didn't Know|Knew It →
For Constant Temperature, Gibbs Free Energy is defined as:

Where ,  is the change in Gibbs Free Energy,
is the change in Gibbs Free Energy,  is the change in enthalpy,
is the change in enthalpy,  is temperature, and
is temperature, and  is the change in entropy.
is the change in entropy.
Which of the following scenarios is not possible?
For Constant Temperature, Gibbs Free Energy is defined as:
Where , 



Which of the following scenarios is not possible?
Tap to reveal answer
The following condition is not possible:

This is because if enthalpy is positive, and entropy is negative, the negative sign in front of the temperature term in the formula becomes positive. Addition of 2 positive numbers can not be negative. Plugging in arbitrary numbers into the other conditions can show they are all possible.
Take the following condition:

Then Gibbs free energy  can either be positive or negative, depending on the magnitude of enthalpy, entropy, and temperature. (If enthalpy is much larger than entropy and temperature, then the difference will be positive, but if entropy *
can either be positive or negative, depending on the magnitude of enthalpy, entropy, and temperature. (If enthalpy is much larger than entropy and temperature, then the difference will be positive, but if entropy *  is greater than the enthalpy, then the difference will be negative).
is greater than the enthalpy, then the difference will be negative).
The following condition is not possible:
This is because if enthalpy is positive, and entropy is negative, the negative sign in front of the temperature term in the formula becomes positive. Addition of 2 positive numbers can not be negative. Plugging in arbitrary numbers into the other conditions can show they are all possible.
Take the following condition:
Then Gibbs free energy 

← Didn't Know|Knew It →
For constant temperature, Gibbs free energy is defined as:

Where ,  is the change in Gibbs free energy,
is the change in Gibbs free energy,  is the change in enthalpy,
is the change in enthalpy,  is temperature, and
is temperature, and 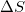 is the change in entropy.
is the change in entropy.
Given that a system is spontaneous, which of the following states are possible?
I. 
II. 
III. 
IV. 
For constant temperature, Gibbs free energy is defined as:
Where , 


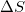
Given that a system is spontaneous, which of the following states are possible?
I.
II.
III.
IV.
Tap to reveal answer
Condition I is always true. Condition II is never true, as Gibbs free energy cannot be negative if enthalpy is positive and entropy is negative. Condition III may be true if temperature is very high (this is the scenario when the  term dominates the
term dominates the  term. Condition IV is not possible because
term. Condition IV is not possible because  and we were given a system with a Gibbs free Energy that is
and we were given a system with a Gibbs free Energy that is  (we were told the system was spontaneous).
(we were told the system was spontaneous).
Condition I is always true. Condition II is never true, as Gibbs free energy cannot be negative if enthalpy is positive and entropy is negative. Condition III may be true if temperature is very high (this is the scenario when the 



← Didn't Know|Knew It →
The enthalpy of a reaction is  and the entropy of a reaction is
and the entropy of a reaction is  . Which of the following is the Gibbs free energy (in
. Which of the following is the Gibbs free energy (in  ) of this reaction?
) of this reaction?
The enthalpy of a reaction is 


Tap to reveal answer
Gibbs free energy of a system can be solved using the following equation.

where  is change in Gibbs free energy,
is change in Gibbs free energy,  is change in enthalpy,
is change in enthalpy,  is temperature in Kelvins and
is temperature in Kelvins and  is change in entropy. To solve for
is change in entropy. To solve for  we need all three of the variables. We are not given the temperature; therefore, we cannot solve for Gibbs free energy.
we need all three of the variables. We are not given the temperature; therefore, we cannot solve for Gibbs free energy.
Gibbs free energy of a system can be solved using the following equation.
where 




← Didn't Know|Knew It →
In an exergonic reaction, products will have Gibbs free energy and the reaction is .
In an exergonic reaction, products will have Gibbs free energy and the reaction is .
Tap to reveal answer
Exergonic reaction suggests that the Gibbs free energy is negative. Since the change in Gibbs free energy is defined as Gibbs free energy of products - Gibbs free energy of reactants, a negative change in Gibbs free energy suggests that the products have a lower Gibbs free energy than reactants. A reaction is spontaneous if it has negative Gibbs free energy; therefore, exergonic reactions are always spontaneous. This is because the reaction is producing a more stable product (lower energy) from a less stable reactant (higher energy).
Exergonic reaction suggests that the Gibbs free energy is negative. Since the change in Gibbs free energy is defined as Gibbs free energy of products - Gibbs free energy of reactants, a negative change in Gibbs free energy suggests that the products have a lower Gibbs free energy than reactants. A reaction is spontaneous if it has negative Gibbs free energy; therefore, exergonic reactions are always spontaneous. This is because the reaction is producing a more stable product (lower energy) from a less stable reactant (higher energy).
← Didn't Know|Knew It →
In an exergonic reaction, products will have Gibbs free energy and the reaction is .
In an exergonic reaction, products will have Gibbs free energy and the reaction is .
Tap to reveal answer
Exergonic reaction suggests that the Gibbs free energy is negative. Since the change in Gibbs free energy is defined as Gibbs free energy of products - Gibbs free energy of reactants, a negative change in Gibbs free energy suggests that the products have a lower Gibbs free energy than reactants. A reaction is spontaneous if it has negative Gibbs free energy; therefore, exergonic reactions are always spontaneous. This is because the reaction is producing a more stable product (lower energy) from a less stable reactant (higher energy).
Exergonic reaction suggests that the Gibbs free energy is negative. Since the change in Gibbs free energy is defined as Gibbs free energy of products - Gibbs free energy of reactants, a negative change in Gibbs free energy suggests that the products have a lower Gibbs free energy than reactants. A reaction is spontaneous if it has negative Gibbs free energy; therefore, exergonic reactions are always spontaneous. This is because the reaction is producing a more stable product (lower energy) from a less stable reactant (higher energy).
← Didn't Know|Knew It →
For Constant Temperature, Gibbs Free Energy is defined as:

Where ,  is the change in Gibbs Free Energy,
is the change in Gibbs Free Energy,  is the change in enthalpy,
is the change in enthalpy,  is temperature, and
is temperature, and  is the change in entropy.
is the change in entropy.
Which of the following scenarios is not possible?
For Constant Temperature, Gibbs Free Energy is defined as:
Where , 



Which of the following scenarios is not possible?
Tap to reveal answer
The following condition is not possible:

This is because if enthalpy is positive, and entropy is negative, the negative sign in front of the temperature term in the formula becomes positive. Addition of 2 positive numbers can not be negative. Plugging in arbitrary numbers into the other conditions can show they are all possible.
Take the following condition:

Then Gibbs free energy  can either be positive or negative, depending on the magnitude of enthalpy, entropy, and temperature. (If enthalpy is much larger than entropy and temperature, then the difference will be positive, but if entropy *
can either be positive or negative, depending on the magnitude of enthalpy, entropy, and temperature. (If enthalpy is much larger than entropy and temperature, then the difference will be positive, but if entropy *  is greater than the enthalpy, then the difference will be negative).
is greater than the enthalpy, then the difference will be negative).
The following condition is not possible:
This is because if enthalpy is positive, and entropy is negative, the negative sign in front of the temperature term in the formula becomes positive. Addition of 2 positive numbers can not be negative. Plugging in arbitrary numbers into the other conditions can show they are all possible.
Take the following condition:
Then Gibbs free energy 

← Didn't Know|Knew It →
For constant temperature, Gibbs free energy is defined as:

Where ,  is the change in Gibbs free energy,
is the change in Gibbs free energy,  is the change in enthalpy,
is the change in enthalpy,  is temperature, and
is temperature, and 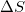 is the change in entropy.
is the change in entropy.
Given that a system is spontaneous, which of the following states are possible?
I. 
II. 
III. 
IV. 
For constant temperature, Gibbs free energy is defined as:
Where , 


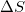
Given that a system is spontaneous, which of the following states are possible?
I.
II.
III.
IV.
Tap to reveal answer
Condition I is always true. Condition II is never true, as Gibbs free energy cannot be negative if enthalpy is positive and entropy is negative. Condition III may be true if temperature is very high (this is the scenario when the  term dominates the
term dominates the  term. Condition IV is not possible because
term. Condition IV is not possible because  and we were given a system with a Gibbs free Energy that is
and we were given a system with a Gibbs free Energy that is  (we were told the system was spontaneous).
(we were told the system was spontaneous).
Condition I is always true. Condition II is never true, as Gibbs free energy cannot be negative if enthalpy is positive and entropy is negative. Condition III may be true if temperature is very high (this is the scenario when the 



← Didn't Know|Knew It →
The enthalpy of a reaction is  and the entropy of a reaction is
and the entropy of a reaction is  . Which of the following is the Gibbs free energy (in
. Which of the following is the Gibbs free energy (in  ) of this reaction?
) of this reaction?
The enthalpy of a reaction is 


Tap to reveal answer
Gibbs free energy of a system can be solved using the following equation.

where  is change in Gibbs free energy,
is change in Gibbs free energy,  is change in enthalpy,
is change in enthalpy,  is temperature in Kelvins and
is temperature in Kelvins and  is change in entropy. To solve for
is change in entropy. To solve for  we need all three of the variables. We are not given the temperature; therefore, we cannot solve for Gibbs free energy.
we need all three of the variables. We are not given the temperature; therefore, we cannot solve for Gibbs free energy.
Gibbs free energy of a system can be solved using the following equation.
where 




← Didn't Know|Knew It →