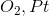Using Other Organic Oxidizing Agents
Help Questions
Organic Chemistry › Using Other Organic Oxidizing Agents
Which of the following reagents can turn primary alcohols into a carboxylic acid?
Jones Reagent (chromic acid in acetone)
Tollen's test
PCC
Explanation
The Jones reagent can convert primary alcohol to acids and secondary alcohols to ketones. The Tollen's test only converts aldehydes to carboxylic acids. PCC can only convert primary and secondary alcohol to aldehydes and ketones, respectively. 

What is an appropriate reagent to convert a primary alcohol to an aldehyde?
Explanation
To form the aldehyde, the alcohol must be oxidized. However, potassium permanganate and chromic acid are too strong and would yield a carboxylic acid. Ozonolysis works with alkenes and oxygen over platinum would not react. PCC is correct because it will oxidize the alcohol to form an aldehyde but is too weak to continue on to form the carboxylic acid.