Organic Functional Groups and Molecules - Organic Chemistry
Card 1 of 344
A researcher wants to convert the given molecule's ketone group into a tertiary alcohol. Select the correct order of steps she must take to produce a tertiary alcohol at the ketone, but leave the aldehyde intact.
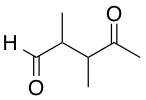
A researcher wants to convert the given molecule's ketone group into a tertiary alcohol. Select the correct order of steps she must take to produce a tertiary alcohol at the ketone, but leave the aldehyde intact.

Tap to reveal answer
An aldehyde is more electrophilic than a ketone, so to do chemistry on the ketone, we must protect the aldehyde. A common protecting group for aldehydes and ketones is ethane-1,2-diol, as it forms a meta-stable five-membered acetal, which can be hydrolyzed to produce the original aldehyde or ketone by applying heat and acid.
As shown in the scheme below, which corresponds to the correct answer choice, once the aldehyde is protected, then the ketone can be reacted with the Grignard MeMgBr reagent to add a methyl group at the carbonyl. An acid workup removes the protecting group to reveal the original aldehyde, and affords the desired tertiary alcohol.

The schemes below illustrate why each of the other answer choices is wrong, as no other sequence will produce the desired product:

An aldehyde is more electrophilic than a ketone, so to do chemistry on the ketone, we must protect the aldehyde. A common protecting group for aldehydes and ketones is ethane-1,2-diol, as it forms a meta-stable five-membered acetal, which can be hydrolyzed to produce the original aldehyde or ketone by applying heat and acid.
As shown in the scheme below, which corresponds to the correct answer choice, once the aldehyde is protected, then the ketone can be reacted with the Grignard MeMgBr reagent to add a methyl group at the carbonyl. An acid workup removes the protecting group to reveal the original aldehyde, and affords the desired tertiary alcohol.

The schemes below illustrate why each of the other answer choices is wrong, as no other sequence will produce the desired product:

← Didn't Know|Knew It →
Which of the following carbocation intermediates requires the least activation energy?
Which of the following carbocation intermediates requires the least activation energy?
Tap to reveal answer
The more stable the carbocation, the lower the activation energy for reaching that intermediate will be. The more substituted a carbocation is, the more stable it is. The carbocation bonded to three alkanes (tertiary carbocation) is the most stable, and thus the correct answer.
Secondary carbocations will require more energy than tertiary, and primary carbocations will require the most energy.
The more stable the carbocation, the lower the activation energy for reaching that intermediate will be. The more substituted a carbocation is, the more stable it is. The carbocation bonded to three alkanes (tertiary carbocation) is the most stable, and thus the correct answer.
Secondary carbocations will require more energy than tertiary, and primary carbocations will require the most energy.
← Didn't Know|Knew It →
Rank the following carbocations from least to most stable.
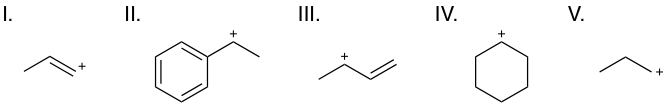
Rank the following carbocations from least to most stable.

Tap to reveal answer
You many know that primary carbocations are less stable than secondary, which are in turn less stable than tertiary carbocations. This holds true in this question, and as such, V < IV. To evaluate compounds I, II, and III, however, we will need to determine their resonance capabilities.
We can compare the two special secondary carbocations, II and III—a benzyl carbocation and an allyl carbocation, respectively. The structure of these cations allow for the positive charge to be shared over several atoms via resonance as shown below:

We can see that the benzyl carbocation (II) is more stable than the allyl carbocation (III), as all its resonance forms have a secondary carbocation, and there are more resonance forms. As both share charge over multiple atoms, both are more stable than IV and V, which place charge on one atom only. Thus far, we can say: V < VI < III < II.
Lastly, we must consider I, a vinylic carbocation. This cation puts its negative charge on an sp2-hybridized carbon, and is the only example of this situation. Because an sp2 orbital has more overall s- character than an sp3 orbital, electrons are held closer to the nucleus. As the electrons feel more positive charge from the nucleus, the atom can accommodate more negative charge in general. We can think of this as a shift towards more electronegative behavior. As such, a positive charge will be more unstable on an sp2-hybridized carbon than on an sp3-hybridized carbon, and we can conclude than I is the least stable of all choices.
Thus, the correct answer is I < V < IV < III < II.
You many know that primary carbocations are less stable than secondary, which are in turn less stable than tertiary carbocations. This holds true in this question, and as such, V < IV. To evaluate compounds I, II, and III, however, we will need to determine their resonance capabilities.
We can compare the two special secondary carbocations, II and III—a benzyl carbocation and an allyl carbocation, respectively. The structure of these cations allow for the positive charge to be shared over several atoms via resonance as shown below:

We can see that the benzyl carbocation (II) is more stable than the allyl carbocation (III), as all its resonance forms have a secondary carbocation, and there are more resonance forms. As both share charge over multiple atoms, both are more stable than IV and V, which place charge on one atom only. Thus far, we can say: V < VI < III < II.
Lastly, we must consider I, a vinylic carbocation. This cation puts its negative charge on an sp2-hybridized carbon, and is the only example of this situation. Because an sp2 orbital has more overall s- character than an sp3 orbital, electrons are held closer to the nucleus. As the electrons feel more positive charge from the nucleus, the atom can accommodate more negative charge in general. We can think of this as a shift towards more electronegative behavior. As such, a positive charge will be more unstable on an sp2-hybridized carbon than on an sp3-hybridized carbon, and we can conclude than I is the least stable of all choices.
Thus, the correct answer is I < V < IV < III < II.
← Didn't Know|Knew It →
Under which reaction mechanism can rearrangements occur?
Under which reaction mechanism can rearrangements occur?
Tap to reveal answer
Rearrangements generally require carbocations. Carbocations are only formed under mechanisms with unimolecular rate-limiting steps (i.e. carbocation formation).  reactions are unimolecular, involve carbocation intermediates, and therefore can undergo rearrangement.
reactions are unimolecular, involve carbocation intermediates, and therefore can undergo rearrangement.
Rearrangements generally require carbocations. Carbocations are only formed under mechanisms with unimolecular rate-limiting steps (i.e. carbocation formation). 
← Didn't Know|Knew It →

How many hydrogens are present in the given molecule?
How many hydrogens are present in the given molecule?
Tap to reveal answer
Each alcohol group provides a hydrogen. Additionally, there are 24 "hidden" hydrogens. Refer to the photo below to see where these "hidden" hydrogens are located.

Each alcohol group provides a hydrogen. Additionally, there are 24 "hidden" hydrogens. Refer to the photo below to see where these "hidden" hydrogens are located.
← Didn't Know|Knew It →

List all functional groups in the given molecule.
List all functional groups in the given molecule.
Tap to reveal answer
The first carbon that is attached to the chlorine is an alkyl halide. The oxygen that is directly attached to two carbons is an ether. Lastly, the  is a carboxylic acid.
is a carboxylic acid.
The first carbon that is attached to the chlorine is an alkyl halide. The oxygen that is directly attached to two carbons is an ether. Lastly, the 
← Didn't Know|Knew It →
What is the general formula of an ether?
What is the general formula of an ether?
Tap to reveal answer
 is the general formula of an ether.
is the general formula of an ether.

← Didn't Know|Knew It →

Which functional groups are present in the pictured molecule?
Which functional groups are present in the pictured molecule?
Tap to reveal answer
This picture shows each functional group in its own box.
1. Phenyl
2. Ketone
3. Amine
4. Acid anhydride

This picture shows each functional group in its own box.
1. Phenyl
2. Ketone
3. Amine
4. Acid anhydride
← Didn't Know|Knew It →
Which of the following compounds has the molecular formula,  ?
?
Which of the following compounds has the molecular formula, 
Tap to reveal answer
The molecular formula  follows the rule regarding the molecular formula of alkenes:
follows the rule regarding the molecular formula of alkenes:  . Therefore,
. Therefore,  is the molecular formula for hexene.
is the molecular formula for hexene.
The molecular formula 


← Didn't Know|Knew It →
What kind of organic compounds contain an  group?
group?
What kind of organic compounds contain an 
Tap to reveal answer
By definition, alcohols are compounds that contain an  (hydroxyl) group bonded covalently to the end of a hydrocarbon.
(hydroxyl) group bonded covalently to the end of a hydrocarbon.
By definition, alcohols are compounds that contain an 
← Didn't Know|Knew It →
Ethanol  is characterized as what kind of alcohol?
is characterized as what kind of alcohol?
Ethanol 
Tap to reveal answer
Ethanol (or ethyl alcohol) is characterized as a primary alcohol since there is only one carbon-containing group that is directly attached to the carbon containing the  group.
group.
Ethanol (or ethyl alcohol) is characterized as a primary alcohol since there is only one carbon-containing group that is directly attached to the carbon containing the 
← Didn't Know|Knew It →
What is the IUPAC name for the compound shown?

What is the IUPAC name for the compound shown?
Tap to reveal answer
The molecule's longest carbon chain has 5 carbons (thus, "pent"), and the one double bond makes it an alkENE (thus "pentene"). The longest chain is a ring structure (thus "cyclopentene"). Because the IUPAC rules automatically assign the location of the first double bond to carbons 1 and 2, there is no need for a number locand.
The molecule's longest carbon chain has 5 carbons (thus, "pent"), and the one double bond makes it an alkENE (thus "pentene"). The longest chain is a ring structure (thus "cyclopentene"). Because the IUPAC rules automatically assign the location of the first double bond to carbons 1 and 2, there is no need for a number locand.
← Didn't Know|Knew It →
What is the IUPAC name for the compound shown?

What is the IUPAC name for the compound shown?
Tap to reveal answer
The molecule's longest carbon chain has 4 carbons (thus, "but"), and the presence of two double bonds makes it an alkENE, more specifically, a diene (thus "butadiene"). Because there is more than one way in which the double bonds can be arranged (between C1-C2 and C2-C3, or between C1-C2 and C3-C4), it's important to place locants indicating the lower-numbered carbon in each double bond.
The molecule's longest carbon chain has 4 carbons (thus, "but"), and the presence of two double bonds makes it an alkENE, more specifically, a diene (thus "butadiene"). Because there is more than one way in which the double bonds can be arranged (between C1-C2 and C2-C3, or between C1-C2 and C3-C4), it's important to place locants indicating the lower-numbered carbon in each double bond.
← Didn't Know|Knew It →
What is the IUPAC name for the molecule shown below?

What is the IUPAC name for the molecule shown below?
Tap to reveal answer
The molecule's longest carbon chain has 5 carbons (thus, "pent-"), and the carbon-carbon double bond makes it an alkENE (thus "pentene"). The location of the double bond must be specified, and numbering the carbon chain to give the double bond the lowest numbers possible mean that it is numbered from right to left, putting the double bond between carbon 2 and carbon 3. This will put the methyl group on carbon 3.
Regarding stereochemistry, on carbon 2, the higher priority substituent is the methyl group. On carbon 3, the ethyl group is the higher priority. The higher priority substituents are on the same side of the double bond, and therefore the stereochemistry designation is "Z."
The molecule's longest carbon chain has 5 carbons (thus, "pent-"), and the carbon-carbon double bond makes it an alkENE (thus "pentene"). The location of the double bond must be specified, and numbering the carbon chain to give the double bond the lowest numbers possible mean that it is numbered from right to left, putting the double bond between carbon 2 and carbon 3. This will put the methyl group on carbon 3.
Regarding stereochemistry, on carbon 2, the higher priority substituent is the methyl group. On carbon 3, the ethyl group is the higher priority. The higher priority substituents are on the same side of the double bond, and therefore the stereochemistry designation is "Z."
← Didn't Know|Knew It →
What is the IUPAC name for the molecule shown?

What is the IUPAC name for the molecule shown?
Tap to reveal answer
The molecule's longest carbon chain has 6 carbons (thus, "hex-"), and the lack of carbon-carbon double bonds makes it an alkANE (thus "hexan-"). The presence of a hydroxyl group makes this molecule an alcohol (thus "hexanol"). The longest carbon chain is a ring structure (thus "cyclohexanol"), and the location of the alcohol group is assumed to be carbon 1 because it's the highest priority functional group on the molecule. The only other substituent is a methyl group, and numbering the carbon chain starting from the one containing the alcohol group and moving toward the methyl group puts the methyl group on carbon 2. Thus "2-methylcyclohexanol."
The molecule's longest carbon chain has 6 carbons (thus, "hex-"), and the lack of carbon-carbon double bonds makes it an alkANE (thus "hexan-"). The presence of a hydroxyl group makes this molecule an alcohol (thus "hexanol"). The longest carbon chain is a ring structure (thus "cyclohexanol"), and the location of the alcohol group is assumed to be carbon 1 because it's the highest priority functional group on the molecule. The only other substituent is a methyl group, and numbering the carbon chain starting from the one containing the alcohol group and moving toward the methyl group puts the methyl group on carbon 2. Thus "2-methylcyclohexanol."
← Didn't Know|Knew It →
What is the IUPAC name for the compound shown?

What is the IUPAC name for the compound shown?
Tap to reveal answer
The molecule's longest carbon chain has 6 carbons (thus, "hex-"), and the presence of three double bonds makes it an alkENE, more specifically, a tri ene (thus "hexatriene"). Because there is more than one way in which the double bonds can be arranged it's important to place locants indicating the lower-numbered carbon in each double bond (1, 3, and 5 in this case).
The molecule's longest carbon chain has 6 carbons (thus, "hex-"), and the presence of three double bonds makes it an alkENE, more specifically, a tri ene (thus "hexatriene"). Because there is more than one way in which the double bonds can be arranged it's important to place locants indicating the lower-numbered carbon in each double bond (1, 3, and 5 in this case).
← Didn't Know|Knew It →
Name this compound by IUPAC rules:

Name this compound by IUPAC rules:

Tap to reveal answer
When naming an organic compound by the IUPAC rules, it's best to first start by identifying the functional groups present.
In this particular case we have:
An Ester in the middle as shown here:

a Methyl group shown here:

and a Butyl group attached to the ester:

Next, we should identify what functional group has the highest priority, as that will form the base name of the compound:
According to IUPAC convention, Carboxylic Acid derivatives including Esters have the highest priority then carbonyls then alcohols, amines, alkenes, alkynes, and alkanes, so in this case the Ester group has the highest priority and therefore makes up the name of the base compound.
Next, we want to number the longest carbon chain with the highest priority functional group with the lowest number. In this case this means we want the carbonyl of the ester to be carbon number #1, so let's start there and number the carbon chain.
You should get something like this:

Notice there are two sixes. The reason why is because there are two possible pathways for the carbon numbering to continue, but both are equivalent meaning no matter what we do there is a 5-methyl group and the carbon chain is 6 carbons long.
Now that we have numbered the carbon chain we can begin our naming.
Let's start with the base name:
According to IUPAC convention the base name for an ester compound is -oate, so in this case we have a hexanoate, which can also be written as hexan-1-oate, but this isn't needed as it as the ester is at carbon 1.
We also have a methyl group at the 5-carbon so in this gives us:
5-methylhexanoate.
However, we aren't done as we haven't named the substituent on the other side of the ester. Let's first count the number of carbons it has. Since this chain has 4 carbons it is a butyl group, as according to IUPAC the chain on the side farthest from carbonyl carbon of the ester is named as a substituent and placed in front of the name of the compound.
This makes our final answer Butyl 5-methylhexanoate.
Now let's go over the other answer choices and why they are wrong:
1) Butyl 5-methylhexenoate is almost correct except for the fact it says Butyl 5-methylhexenoate. The "en" indicates there is an alkene (double bond) in the compound, and since there isn't this can't be the right answer.
2) Butyl 2-methylhex-6-anoate is wrong because the ester group isn't assigned the highest priority. In IUPAC nomenclature you want to assign the highest priority functional group the lowest number possible in the carbon chain.
3) Butyl 2-methylhex-6-enoate is wrong for a mix of the reasons in the previous 2 answers. It says Butyl 2-methylhex-6-enoate in it, and the compound doesn't have an alkene. It also makes the mistake of not making the ester group (the highest priority functional group) have the lowest number possible in the carbon chain, so this can't be right either.
4) 1-butoxy-5-methylhexanone is wrong because it interprets the ester as being a ketone and an ether group instead of an ester.
When naming an organic compound by the IUPAC rules, it's best to first start by identifying the functional groups present.
In this particular case we have:
An Ester in the middle as shown here:

a Methyl group shown here:

and a Butyl group attached to the ester:

Next, we should identify what functional group has the highest priority, as that will form the base name of the compound:
According to IUPAC convention, Carboxylic Acid derivatives including Esters have the highest priority then carbonyls then alcohols, amines, alkenes, alkynes, and alkanes, so in this case the Ester group has the highest priority and therefore makes up the name of the base compound.
Next, we want to number the longest carbon chain with the highest priority functional group with the lowest number. In this case this means we want the carbonyl of the ester to be carbon number #1, so let's start there and number the carbon chain.
You should get something like this:

Notice there are two sixes. The reason why is because there are two possible pathways for the carbon numbering to continue, but both are equivalent meaning no matter what we do there is a 5-methyl group and the carbon chain is 6 carbons long.
Now that we have numbered the carbon chain we can begin our naming.
Let's start with the base name:
According to IUPAC convention the base name for an ester compound is -oate, so in this case we have a hexanoate, which can also be written as hexan-1-oate, but this isn't needed as it as the ester is at carbon 1.
We also have a methyl group at the 5-carbon so in this gives us:
5-methylhexanoate.
However, we aren't done as we haven't named the substituent on the other side of the ester. Let's first count the number of carbons it has. Since this chain has 4 carbons it is a butyl group, as according to IUPAC the chain on the side farthest from carbonyl carbon of the ester is named as a substituent and placed in front of the name of the compound.
This makes our final answer Butyl 5-methylhexanoate.
Now let's go over the other answer choices and why they are wrong:
1) Butyl 5-methylhexenoate is almost correct except for the fact it says Butyl 5-methylhexenoate. The "en" indicates there is an alkene (double bond) in the compound, and since there isn't this can't be the right answer.
2) Butyl 2-methylhex-6-anoate is wrong because the ester group isn't assigned the highest priority. In IUPAC nomenclature you want to assign the highest priority functional group the lowest number possible in the carbon chain.
3) Butyl 2-methylhex-6-enoate is wrong for a mix of the reasons in the previous 2 answers. It says Butyl 2-methylhex-6-enoate in it, and the compound doesn't have an alkene. It also makes the mistake of not making the ester group (the highest priority functional group) have the lowest number possible in the carbon chain, so this can't be right either.
4) 1-butoxy-5-methylhexanone is wrong because it interprets the ester as being a ketone and an ether group instead of an ester.
← Didn't Know|Knew It →
Name this compound according to IUPAC naming convention:

Name this compound according to IUPAC naming convention:

Tap to reveal answer
When naming an organic compound by the IUPAC, it's best to first start by identifying the functional groups present.
In this particular case we have:
A carboxylic acid shown here:

An alkene in the middle of the carbon chain:

A methyl group:

and a ketone towards the end:

Next, we should identify what functional group has the highest priority, as that will form the base name of the compound:
According to IUPAC convention, Carboxylic Acid derivatives including Esters have the highest priority then carbonyls (in this case the ketone) then alcohols, amines, alkenes, alkynes, and alkanes, so in this case the Ester group has the highest priority and therefore makes up the name of the base compound.
Next, we want to number the longest carbon chain with the highest priority functional group with the lowest number. In this case this means we want the carbonyl of the carboxylic acid to be carbon number #1, so let's start there and number the carbon chain.
You should get something like this:

Now that we have numbered the carbon chain we can begin our naming.
Let's start with the base name:
According to IUPAC convention the base name for an carboxylic acid compound is -oic acid, so in this case we have a decanoic acid, which can also be written as decan-1-oic acid, but this isn't needed as it as the carboxylic acid is at carbon 1.
Next notice that we have an alkene that's part of the main chain, since it is part of the main chain we include it in the base name, so we must change our name from decanoic acid to dec**-4-en**oic acid because the lowest it can be numbered is #4, however since the highest priority groups on the alkene are facing opposite to each other it is an E (Entgegen) alkene, so we can name it (4E)-decenoic acid
We also have a methyl group at carbon 5. This gives us:
(4E)-5-methyldecenoic acid.
Finally we have a ketone as a substituent, and a ketone as a substituent is called an oxo, so it becomes 9-oxo.
Now we must order our substituents alphabetically. Thus it becomes
(4E)-5-methyl-9-oxodecenoic acid which is our final answer.
Now let's go over the wrong answers:
-
(4E)-9-oxo-5-methyldecenoic acid is wrong because the substituents aren't ordered alphabetically.
-
5-methyl-9-oxodecanoic acid is wrong because it says decanoic acid when there is an alkene present.
-
9-oxo-5-methyldecanoic acid is wrong because it says decanoic acid when there is an alkene present, and because the substituents aren't ordered alphabetically.
-
10-carboxy-5-methyldecan-2-one is wrong because the carboxylic acid group isn't highest priority and it omits the alkene in this compound.
When naming an organic compound by the IUPAC, it's best to first start by identifying the functional groups present.
In this particular case we have:
A carboxylic acid shown here:

An alkene in the middle of the carbon chain:

A methyl group:

and a ketone towards the end:

Next, we should identify what functional group has the highest priority, as that will form the base name of the compound:
According to IUPAC convention, Carboxylic Acid derivatives including Esters have the highest priority then carbonyls (in this case the ketone) then alcohols, amines, alkenes, alkynes, and alkanes, so in this case the Ester group has the highest priority and therefore makes up the name of the base compound.
Next, we want to number the longest carbon chain with the highest priority functional group with the lowest number. In this case this means we want the carbonyl of the carboxylic acid to be carbon number #1, so let's start there and number the carbon chain.
You should get something like this:

Now that we have numbered the carbon chain we can begin our naming.
Let's start with the base name:
According to IUPAC convention the base name for an carboxylic acid compound is -oic acid, so in this case we have a decanoic acid, which can also be written as decan-1-oic acid, but this isn't needed as it as the carboxylic acid is at carbon 1.
Next notice that we have an alkene that's part of the main chain, since it is part of the main chain we include it in the base name, so we must change our name from decanoic acid to dec**-4-en**oic acid because the lowest it can be numbered is #4, however since the highest priority groups on the alkene are facing opposite to each other it is an E (Entgegen) alkene, so we can name it (4E)-decenoic acid
We also have a methyl group at carbon 5. This gives us:
(4E)-5-methyldecenoic acid.
Finally we have a ketone as a substituent, and a ketone as a substituent is called an oxo, so it becomes 9-oxo.
Now we must order our substituents alphabetically. Thus it becomes
(4E)-5-methyl-9-oxodecenoic acid which is our final answer.
Now let's go over the wrong answers:
-
(4E)-9-oxo-5-methyldecenoic acid is wrong because the substituents aren't ordered alphabetically.
-
5-methyl-9-oxodecanoic acid is wrong because it says decanoic acid when there is an alkene present.
-
9-oxo-5-methyldecanoic acid is wrong because it says decanoic acid when there is an alkene present, and because the substituents aren't ordered alphabetically.
-
10-carboxy-5-methyldecan-2-one is wrong because the carboxylic acid group isn't highest priority and it omits the alkene in this compound.
← Didn't Know|Knew It →

What type of orbital is the lone pair on the nitrogen found in?
What type of orbital is the lone pair on the nitrogen found in?
Tap to reveal answer
In this molecule, the carbon that is directly attached to the nitrogen and the nitrogen itself are sp2 hybridized. This means that, within this bond, there are three sp2 orbitals present and the lone pair on the nitrogen occupies one of them.
In this molecule, the carbon that is directly attached to the nitrogen and the nitrogen itself are sp2 hybridized. This means that, within this bond, there are three sp2 orbitals present and the lone pair on the nitrogen occupies one of them.
← Didn't Know|Knew It →

How many conjugated atoms are present in the given molecule?
How many conjugated atoms are present in the given molecule?
Tap to reveal answer
A conjugated molecule is one that has its atoms arranged in such a way that allows p orbitals to lie parallel to each other. This allows for electron delocalization, which ultimately provides increased stability for the molecule. To find conjugated atoms, look for alternating single and multiple bonds. The circled carbon atoms are the only ones that are conjugated in this molecule.

A conjugated molecule is one that has its atoms arranged in such a way that allows p orbitals to lie parallel to each other. This allows for electron delocalization, which ultimately provides increased stability for the molecule. To find conjugated atoms, look for alternating single and multiple bonds. The circled carbon atoms are the only ones that are conjugated in this molecule.
← Didn't Know|Knew It →