Oxidation-Reduction - MCAT Chemical and Physical Foundations of Biological Systems
Card 1 of 175
Consider the half reaction below.
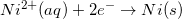

Which of the following statements is true about the oxidation of nickel?
Consider the half reaction below.
Which of the following statements is true about the oxidation of nickel?
Tap to reveal answer
Reduction potentials are typically provided in table form. Since the oxidation potential of an element is the negative of the reduction potential, it can be determined by viewing a reduction table.
Since the reduction potential for nickel is given as –0.23V, we can conclude that the oxidation potential for nickel is 0.23V.
Reduction potentials are typically provided in table form. Since the oxidation potential of an element is the negative of the reduction potential, it can be determined by viewing a reduction table.
Since the reduction potential for nickel is given as –0.23V, we can conclude that the oxidation potential for nickel is 0.23V.
← Didn't Know|Knew It →
The standard reduction potentials for iron and nickel are as follows:




If a galvanic cell is made using these two metals, what is the standard voltage for the cell?
The standard reduction potentials for iron and nickel are as follows:


If a galvanic cell is made using these two metals, what is the standard voltage for the cell?
Tap to reveal answer
A galvanic cell is spontaneous, meaning that there will be a positive cell voltage. In order for this to take place, the more negative reduction potential must be flipped so that the metal is oxidized.




Adding these values together gives a standard cell voltage of  .
.
A galvanic cell is spontaneous, meaning that there will be a positive cell voltage. In order for this to take place, the more negative reduction potential must be flipped so that the metal is oxidized.


Adding these values together gives a standard cell voltage of 
← Didn't Know|Knew It →
Consider the redox reaction:
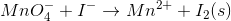
What is the balanced equation for the above redox reaction in acidic solution?
Consider the redox reaction:
What is the balanced equation for the above redox reaction in acidic solution?
Tap to reveal answer

1. Determine the oxidation number of each element.
Reactants: 
Products: 
2. Write and balance the half reactions.
Oxidation (loss of electrons): 
Reduction (gain of electrons): 
3. Balance oxygen by adding water (from the solution) into the half reactions.
Oxidation: 
Reduction: 
4. Balance hydrogen by adding  ions from the acid into the half reactions.
ions from the acid into the half reactions.
Oxidation: 
Reduction: 
5. Multiply each half reaction times an integer such that the electrons cancel when the equations are added. The oxidation reaction will be multiplied by five, and the reduction reaction will be multiplied by two.
Oxidation times 5: 
Reduction times 2: 
6. Add the half reactions together. The electrons will cancel from both the reactants and products.

1. Determine the oxidation number of each element.
Reactants:
Products:
2. Write and balance the half reactions.
Oxidation (loss of electrons):
Reduction (gain of electrons):
3. Balance oxygen by adding water (from the solution) into the half reactions.
Oxidation:
Reduction:
4. Balance hydrogen by adding 
Oxidation:
Reduction:
5. Multiply each half reaction times an integer such that the electrons cancel when the equations are added. The oxidation reaction will be multiplied by five, and the reduction reaction will be multiplied by two.
Oxidation times 5:
Reduction times 2:
6. Add the half reactions together. The electrons will cancel from both the reactants and products.
← Didn't Know|Knew It →
Consider the half reaction below.
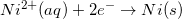

Which of the following statements is true about the oxidation of nickel?
Consider the half reaction below.
Which of the following statements is true about the oxidation of nickel?
Tap to reveal answer
Reduction potentials are typically provided in table form. Since the oxidation potential of an element is the negative of the reduction potential, it can be determined by viewing a reduction table.
Since the reduction potential for nickel is given as –0.23V, we can conclude that the oxidation potential for nickel is 0.23V.
Reduction potentials are typically provided in table form. Since the oxidation potential of an element is the negative of the reduction potential, it can be determined by viewing a reduction table.
Since the reduction potential for nickel is given as –0.23V, we can conclude that the oxidation potential for nickel is 0.23V.
← Didn't Know|Knew It →
The standard reduction potentials for iron and nickel are as follows:




If a galvanic cell is made using these two metals, what is the standard voltage for the cell?
The standard reduction potentials for iron and nickel are as follows:


If a galvanic cell is made using these two metals, what is the standard voltage for the cell?
Tap to reveal answer
A galvanic cell is spontaneous, meaning that there will be a positive cell voltage. In order for this to take place, the more negative reduction potential must be flipped so that the metal is oxidized.




Adding these values together gives a standard cell voltage of  .
.
A galvanic cell is spontaneous, meaning that there will be a positive cell voltage. In order for this to take place, the more negative reduction potential must be flipped so that the metal is oxidized.


Adding these values together gives a standard cell voltage of 
← Didn't Know|Knew It →
Which of the following is the oxidizing agent in the following equation?

Which of the following is the oxidizing agent in the following equation?
Tap to reveal answer
An oxidizing agent is one that oxidizes another element and, becomes reduced in the process. Oxidizing and reducing agents are found in the reactants of the equation, eliminating Al3+ and Cr(s) as answer choices. Cr3+ gains three electrons to become Cr(s), which has an oxidation number of zero. Cr3+ becomes reduced, while Al(s) is oxidized, therefore, Cr3+ is the oxidizing agent. Remember "OIL RIG": Oxidation Is Loss of electrons and Reduction Is Gain of electrons.
An oxidizing agent is one that oxidizes another element and, becomes reduced in the process. Oxidizing and reducing agents are found in the reactants of the equation, eliminating Al3+ and Cr(s) as answer choices. Cr3+ gains three electrons to become Cr(s), which has an oxidation number of zero. Cr3+ becomes reduced, while Al(s) is oxidized, therefore, Cr3+ is the oxidizing agent. Remember "OIL RIG": Oxidation Is Loss of electrons and Reduction Is Gain of electrons.
← Didn't Know|Knew It →

In the above unbalanced redox reaction, which best describes the oxidation/reduction roles of iron and oxygen?
In the above unbalanced redox reaction, which best describes the oxidation/reduction roles of iron and oxygen?
Tap to reveal answer
There are a couple options to solve this problem. First, we could simply remember that anything which combines with oxygen in a redox reaction is oxidized, and the associated oxygen is reduced.
Another option is to think about the oxidation numbers of each element (an element whose oxidation number increases is oxidized, and one whose oxidation number decreases is reduced). If we choose this method, recall that free elements (Fe and O2 in this case) have oxidation number 0. Also, oxygen in compounds has oxidation number -2. Oxygen's oxidation number decreases from 0 to -2, meaning that oxygen is reduced. Since oxidation and reduction occur at the same time in different elements of the redox reaction, iron's oxidation number must increase, so iron is oxidized.
There are a couple options to solve this problem. First, we could simply remember that anything which combines with oxygen in a redox reaction is oxidized, and the associated oxygen is reduced.
Another option is to think about the oxidation numbers of each element (an element whose oxidation number increases is oxidized, and one whose oxidation number decreases is reduced). If we choose this method, recall that free elements (Fe and O2 in this case) have oxidation number 0. Also, oxygen in compounds has oxidation number -2. Oxygen's oxidation number decreases from 0 to -2, meaning that oxygen is reduced. Since oxidation and reduction occur at the same time in different elements of the redox reaction, iron's oxidation number must increase, so iron is oxidized.
← Didn't Know|Knew It →
Consider the following combustion reaction.

Which of the following statements correctly describes carbon in the reaction?
Consider the following combustion reaction.
Which of the following statements correctly describes carbon in the reaction?
Tap to reveal answer
This problem requires us to determine the oxidation number of carbon as a reactant and as a product. The oxidation number of carbon as a reactant is –4, because it is attached to four hydrogens each with a charge of +1. The overall charge on the molecule is zero, the carbon must cancel out the charges contributed by hydrogen.
Carbon as a product has an oxidation number of +4, because it is attached to two oxygens, each with an oxidation number of –2. Again, we know that the molecule is neutral, and carbon must balance the charges from oxygen
Since the oxidation number of carbon went from –4 to +4, we conclude that carbon has been oxidized in the reaction. Any atom that loses electrons is oxidized, while any atom to gain electrons is reduced.
This problem requires us to determine the oxidation number of carbon as a reactant and as a product. The oxidation number of carbon as a reactant is –4, because it is attached to four hydrogens each with a charge of +1. The overall charge on the molecule is zero, the carbon must cancel out the charges contributed by hydrogen.
Carbon as a product has an oxidation number of +4, because it is attached to two oxygens, each with an oxidation number of –2. Again, we know that the molecule is neutral, and carbon must balance the charges from oxygen
Since the oxidation number of carbon went from –4 to +4, we conclude that carbon has been oxidized in the reaction. Any atom that loses electrons is oxidized, while any atom to gain electrons is reduced.
← Didn't Know|Knew It →
Consider the following reaction.

Which of the following atoms is oxidized in the reaction?
Consider the following reaction.
Which of the following atoms is oxidized in the reaction?
Tap to reveal answer
The atom that is oxidized will have a higher oxidation number as a reactant than as a product. Since reactant phosphorus (P4) is in elemental form, it has an oxidation number of 0. As a product, phosphorus (PO43-) has an oxidation number of +5.
Total charge = (P) + 4(O) = –3
–3 = (P) + 4(–2) = (P) + (–8)
p = +5
Because it is now more positive, we say that the phosphorus has been oxidized.
The atom that is oxidized will have a higher oxidation number as a reactant than as a product. Since reactant phosphorus (P4) is in elemental form, it has an oxidation number of 0. As a product, phosphorus (PO43-) has an oxidation number of +5.
Total charge = (P) + 4(O) = –3
–3 = (P) + 4(–2) = (P) + (–8)
p = +5
Because it is now more positive, we say that the phosphorus has been oxidized.
← Didn't Know|Knew It →
Consider the following reaction.

What is the oxidizing reagent in the reaction?
Consider the following reaction.
What is the oxidizing reagent in the reaction?
Tap to reveal answer
The oxidizing reagent is the reactant, not the atom, that is responsible for receiving electrons from another atom in order to oxidize it. The oxygen gas goes from having an oxidation number of 0 to –2. This means that oxygen gas is reduced, but because it oxidized another atom by taking its electrons, we call it the oxidizing reagent.
Reactant oxygen is elemental, and has oxidation number 0.
Product oxygen is in H2O, with net charge of 0.
Total charge = 2(H) + (O) = 0
2(+1) + (O) = 2 + (O) = 0
O = –2
Decrease in oxidation number indicates reduction, and also identifies the oxidizing agent.
The oxidizing reagent is the reactant, not the atom, that is responsible for receiving electrons from another atom in order to oxidize it. The oxygen gas goes from having an oxidation number of 0 to –2. This means that oxygen gas is reduced, but because it oxidized another atom by taking its electrons, we call it the oxidizing reagent.
Reactant oxygen is elemental, and has oxidation number 0.
Product oxygen is in H2O, with net charge of 0.
Total charge = 2(H) + (O) = 0
2(+1) + (O) = 2 + (O) = 0
O = –2
Decrease in oxidation number indicates reduction, and also identifies the oxidizing agent.
← Didn't Know|Knew It →
Consider the following oxidation-reduction reaction.

Which of the following statements is true?
Consider the following oxidation-reduction reaction.
Which of the following statements is true?
Tap to reveal answer
In the reaction, solid potassium (K) is initially in elemental form, so it has an oxidation state of 0. The potassium ions (K+) have a charge of +1 in the product. Since the potassium has lost an electron, it has been oxidized.
Since each hydrogen in reactants (NH3) has an oxidation state of +1, and the hydrogen gas (H2) has a charge of 0 as a product, the hydrogen has been reduced. Since it was reduced, we conclude that NH3 is the oxidizing reagent for the reaction.
In the reaction, solid potassium (K) is initially in elemental form, so it has an oxidation state of 0. The potassium ions (K+) have a charge of +1 in the product. Since the potassium has lost an electron, it has been oxidized.
Since each hydrogen in reactants (NH3) has an oxidation state of +1, and the hydrogen gas (H2) has a charge of 0 as a product, the hydrogen has been reduced. Since it was reduced, we conclude that NH3 is the oxidizing reagent for the reaction.
← Didn't Know|Knew It →
Which compound is oxidized in the following reaction?

Which compound is oxidized in the following reaction?
Tap to reveal answer
Oxidation is defined by a loss of electrons, generally resulting in a more positive charge on an atom. Reduction is defined by a gain of electrons, generally resulting in a more negative charge on an atom. To identify the compound being oxidized, we need to find which compound is becoming more positive.

Zinc is initially neutral, but gains a positive two charge as a product. Hydrogen initially has one positive charge, but becomes neutral in the product. The distinction becomes even clearer if we break the overall reaction into two half-reactions.


We can see that zinc is losing electrons and hydrogen is gaining electrons. Zinc is thus being oxidized.
Oxidation is defined by a loss of electrons, generally resulting in a more positive charge on an atom. Reduction is defined by a gain of electrons, generally resulting in a more negative charge on an atom. To identify the compound being oxidized, we need to find which compound is becoming more positive.
Zinc is initially neutral, but gains a positive two charge as a product. Hydrogen initially has one positive charge, but becomes neutral in the product. The distinction becomes even clearer if we break the overall reaction into two half-reactions.
We can see that zinc is losing electrons and hydrogen is gaining electrons. Zinc is thus being oxidized.
← Didn't Know|Knew It →
Consider the combustion of cyclohexane in air at 298K to give gaseous carbon dioxide and liquid water, as shown in this reaction.

In this reaction, is the oxidizing agent and is the reducing agent.
Consider the combustion of cyclohexane in air at 298K to give gaseous carbon dioxide and liquid water, as shown in this reaction.
In this reaction, is the oxidizing agent and is the reducing agent.
Tap to reveal answer
In this reaction, the oxidation number of oxygen goes from  , in diatomic gaseous oxygen, to
, in diatomic gaseous oxygen, to  , in both carbon dioxide and water. This indicates that it has gained electrons; a gain of electrons indicates that oxygen has been reduced. Since it is reduced, it is the oxidizing agent.
, in both carbon dioxide and water. This indicates that it has gained electrons; a gain of electrons indicates that oxygen has been reduced. Since it is reduced, it is the oxidizing agent.
The oxidation number of carbon goes from  in cyclohexane to
in cyclohexane to  in carbon dioxide. This indicates that it has lost electrons; a loss of electrons indicates that carbon has been oxidized. Since it is oxidized, cyclohexane is the reducing agent.
in carbon dioxide. This indicates that it has lost electrons; a loss of electrons indicates that carbon has been oxidized. Since it is oxidized, cyclohexane is the reducing agent.
In this reaction, the oxidation number of oxygen goes from 

The oxidation number of carbon goes from 

← Didn't Know|Knew It →
Which of the following is an incorrect example of a combustion reaction?
Which of the following is an incorrect example of a combustion reaction?
Tap to reveal answer
We often hear of combustion reactions producing carbon dioxide and water. This is true when the reactants contain all three elements (hydrogen, oxygen, and carbon) needed to make carbon dioxide and water. The trick here is to understand that although combustion is just an oxidation reaction (ie, creating more carbon-oxygen bonds), all reactions must still be balanced. One answer choice fails to balance hydrogen in the reaction.

Although we do see an oxidation reaction with the formation of carbon dioxide, the reaction is NOT balanced. This reaction cannot possibly take place as written.
We often hear of combustion reactions producing carbon dioxide and water. This is true when the reactants contain all three elements (hydrogen, oxygen, and carbon) needed to make carbon dioxide and water. The trick here is to understand that although combustion is just an oxidation reaction (ie, creating more carbon-oxygen bonds), all reactions must still be balanced. One answer choice fails to balance hydrogen in the reaction.
Although we do see an oxidation reaction with the formation of carbon dioxide, the reaction is NOT balanced. This reaction cannot possibly take place as written.
← Didn't Know|Knew It →
The Haber-Bosch process, or simply the Haber process, is a common industrial reaction that generates ammonia from nitrogen and hydrogen gas. A worker in a company generates ammonia from the Haber process. He then dissociates the gaseous ammonia in water to produce an aqueous solution. Since ammonia is a base, it will accept a proton from water, generating  and ammonium ion products. The two reactions involved are:
and ammonium ion products. The two reactions involved are:


A redox (reduction and oxidation) reaction involves a change in the oxidation state of atoms. Oxidation of an atom involves an increase in the oxidation state, whereas reduction involves a decrease in the oxidation state. A oxidized atom will usually become .
The Haber-Bosch process, or simply the Haber process, is a common industrial reaction that generates ammonia from nitrogen and hydrogen gas. A worker in a company generates ammonia from the Haber process. He then dissociates the gaseous ammonia in water to produce an aqueous solution. Since ammonia is a base, it will accept a proton from water, generating 
A redox (reduction and oxidation) reaction involves a change in the oxidation state of atoms. Oxidation of an atom involves an increase in the oxidation state, whereas reduction involves a decrease in the oxidation state. A oxidized atom will usually become .
Tap to reveal answer
The question states that an oxidized atom will increase its oxidation state, which means that the atom will lose electrons and become more positively charged. Since the atom will lose electrons, the number of electrons will be less than the number of protons. Electrons are negatively charged and protons are positively charged; therefore, the presence of more protons in an oxidized atom will make the atom more positive. An oxidized atom will usually become a cation because its number of electrons will decrease.
Reduced atoms will gain electrons and become more negatively charged (decrease oxidation state). Notice that the reactions given in the passage are not redox reactions because the atoms in both reactions retain their oxidation states.
The question states that an oxidized atom will increase its oxidation state, which means that the atom will lose electrons and become more positively charged. Since the atom will lose electrons, the number of electrons will be less than the number of protons. Electrons are negatively charged and protons are positively charged; therefore, the presence of more protons in an oxidized atom will make the atom more positive. An oxidized atom will usually become a cation because its number of electrons will decrease.
Reduced atoms will gain electrons and become more negatively charged (decrease oxidation state). Notice that the reactions given in the passage are not redox reactions because the atoms in both reactions retain their oxidation states.
← Didn't Know|Knew It →
What is the oxidation state of sulfur in H2SO4?
What is the oxidation state of sulfur in H2SO4?
Tap to reveal answer
Since there is no overall charge on the compound the oxidation states must cancel out. Although the oxidation state of hydrides are +1, there are two in the compound that must be accounted for. The same is true for oxygen; although the oxidation number of oxygen is -2, there are four oxygens present accounting for a total of -8. The +2 state contribution from the hydrides and -8 from the oxygens results in a -6 charge. The oxidation state on sulfur must be +6 for the molecule to be neutral.
Since there is no overall charge on the compound the oxidation states must cancel out. Although the oxidation state of hydrides are +1, there are two in the compound that must be accounted for. The same is true for oxygen; although the oxidation number of oxygen is -2, there are four oxygens present accounting for a total of -8. The +2 state contribution from the hydrides and -8 from the oxygens results in a -6 charge. The oxidation state on sulfur must be +6 for the molecule to be neutral.
← Didn't Know|Knew It →
What is the oxidation number of chromium in calcium dichromate (CaCr2O7)?
What is the oxidation number of chromium in calcium dichromate (CaCr2O7)?
Tap to reveal answer
Since the calcium ion has a charge of +2, the dichromate anion must have a charge of -2. Oxygen always has an oxidation number of -2, so the seven oxygens in the molecule have a total number of -14. That leaves a charge of +12 that the two chromiums must balance out (-2 - (-14) = 12). Each chromium must therefore have an oxidation number of +6.
Since the calcium ion has a charge of +2, the dichromate anion must have a charge of -2. Oxygen always has an oxidation number of -2, so the seven oxygens in the molecule have a total number of -14. That leaves a charge of +12 that the two chromiums must balance out (-2 - (-14) = 12). Each chromium must therefore have an oxidation number of +6.
← Didn't Know|Knew It →
What is the oxidation state of nitrogen in HNO3?
What is the oxidation state of nitrogen in HNO3?
Tap to reveal answer
When applying oxidation numbers there are certain hierarchical rules that must be followed.
1. The sum of oxidation states of all the elements in a molecule must add up to the overall charge.
2. Group 1 and Group 2 elements have +1 and +2 oxidation states, respectively.
3. Fluorine has an oxidation state of –1.
4. Hydrogen has an oxidation state of +1 (except in metal hydrides).
5. Oxygen has an oxidation state of –2.
6. Elements of the same group (excluding transition metals) generally have the same oxidation state.
When applying the oxidation state to HNO3, hydrogen has a +1 oxidation state, and each of the oxygen molecules has a –2 oxidation state. Since there is only one hydrogen molecule and three oxygen molecules, the oxidation state of nitrogen must balance out the charge of the hydrogen molecule and oxygen molecules combined.



Nitrogen must have a +5 oxidation state.
When applying oxidation numbers there are certain hierarchical rules that must be followed.
1. The sum of oxidation states of all the elements in a molecule must add up to the overall charge.
2. Group 1 and Group 2 elements have +1 and +2 oxidation states, respectively.
3. Fluorine has an oxidation state of –1.
4. Hydrogen has an oxidation state of +1 (except in metal hydrides).
5. Oxygen has an oxidation state of –2.
6. Elements of the same group (excluding transition metals) generally have the same oxidation state.
When applying the oxidation state to HNO3, hydrogen has a +1 oxidation state, and each of the oxygen molecules has a –2 oxidation state. Since there is only one hydrogen molecule and three oxygen molecules, the oxidation state of nitrogen must balance out the charge of the hydrogen molecule and oxygen molecules combined.
Nitrogen must have a +5 oxidation state.
← Didn't Know|Knew It →
What is the oxidation number of hydrogen in NaH?
What is the oxidation number of hydrogen in NaH?
Tap to reveal answer
When applying oxidation numbers there are certain hierarchical rules that must be followed.
1. The sum of oxidation states of all the elements in a molecule must add up to the overall charge.
2. Group 1 and Group 2 elements have +1 and +2 oxidation states, respectively.
3. Fluorine has an oxidation state of –1.
4. Hydrogen has an oxidation state of +1 (except in metal hydrides).
5. Oxygen has an oxidation state of –2.
6. Elements of the same group (excluding transition metals) generally have the same oxidation state.
Following the oxidation rules, group 1 elements must have an oxidation number of +1.


To balance out the overall charge of zero, the hydrogen in this compound must have an oxidation state of –1. Note that NaH is a metal hydride; therefore, the oxidation state of hydrogen is not necessarily +1.
When applying oxidation numbers there are certain hierarchical rules that must be followed.
1. The sum of oxidation states of all the elements in a molecule must add up to the overall charge.
2. Group 1 and Group 2 elements have +1 and +2 oxidation states, respectively.
3. Fluorine has an oxidation state of –1.
4. Hydrogen has an oxidation state of +1 (except in metal hydrides).
5. Oxygen has an oxidation state of –2.
6. Elements of the same group (excluding transition metals) generally have the same oxidation state.
Following the oxidation rules, group 1 elements must have an oxidation number of +1.
To balance out the overall charge of zero, the hydrogen in this compound must have an oxidation state of –1. Note that NaH is a metal hydride; therefore, the oxidation state of hydrogen is not necessarily +1.
← Didn't Know|Knew It →
What is the correct oxidation number of zinc in the compound  ?
?
What is the correct oxidation number of zinc in the compound 
Tap to reveal answer
The sum of oxidation numbers of each element in this compound must add to the total charge of -2. Hydrogen always has an oxidation number of +1, and oxygen always has an oxidation number of -2.
So, in total for oxygen we have (4) * (-2) = -8, and for hydrogen we have (4) * (+1) = +4.
Combining the oxygen and hydrogen, (+4) + (-8) = -4, so we need an additional +2 to achieve the total charge of -2. Zn must provide this balancing charge, and have an oxidation number of +2.
The sum of oxidation numbers of each element in this compound must add to the total charge of -2. Hydrogen always has an oxidation number of +1, and oxygen always has an oxidation number of -2.
So, in total for oxygen we have (4) * (-2) = -8, and for hydrogen we have (4) * (+1) = +4.
Combining the oxygen and hydrogen, (+4) + (-8) = -4, so we need an additional +2 to achieve the total charge of -2. Zn must provide this balancing charge, and have an oxidation number of +2.
← Didn't Know|Knew It →