Thermodynamic Systems and Calorimetry - MCAT Chemical and Physical Foundations of Biological Systems
Card 1 of 98


In the above reaction, how much heat will be released if 74.0g of sulfur reacts with excess oxygen? Round to the nearest 10kJ.

In the above reaction, how much heat will be released if 74.0g of sulfur reacts with excess oxygen? Round to the nearest 10kJ.
Tap to reveal answer
Basically, this is a unit conversion problem. Starting with grams of sulfur, convert to moles of sulfur, and finally to kJ of heat released. Note that the given enthalpy of reaction,  , is the amount of heat released when two moles of sulfur react with three moles of oxygen.
, is the amount of heat released when two moles of sulfur react with three moles of oxygen.

Note that a negative enthalpy of reaction means the process is exothermic and releases heat, while a positive enthalpy of reaction would mean the process is endothermic and absorbs heat.
Basically, this is a unit conversion problem. Starting with grams of sulfur, convert to moles of sulfur, and finally to kJ of heat released. Note that the given enthalpy of reaction, 
Note that a negative enthalpy of reaction means the process is exothermic and releases heat, while a positive enthalpy of reaction would mean the process is endothermic and absorbs heat.
← Didn't Know|Knew It →
A scientist prepares an experiment to demonstrate the second law of thermodynamics for a chemistry class. In order to conduct the experiment, the scientist brings the class outside in January and gathers a cup of water and a portable stove.
The temperature outside is –10 degrees Celsius. The scientist asks the students to consider the following when answering his questions:
Gibbs Free Energy Formula:
ΔG = ΔH – TΔS
Liquid-Solid Water Phase Change Reaction:
H2O(l) ⇌ H2O(s) + X
The scientist prepares two scenarios.
Scenario 1:
The scientist buries the cup of water outside in the snow, returns to the classroom with his class for one hour, and the class then checks on the cup. They find that the water has frozen in the cup.
Scenario 2:
The scientist then places the frozen cup of water on the stove and starts the gas. The class finds that the water melts quickly.
After the water melts, the scientist asks the students to consider two hypothetical scenarios as a thought experiment.
Scenario 3:
Once the liquid water at the end of scenario 2 melts completely, the scientist turns off the gas and monitors what happens to the water. Despite being in the cold air, the water never freezes.
Scenario 4:
The scientist takes the frozen water from the end of scenario 1, puts it on the active stove, and the water remains frozen.
In this situation described in the passage, which of the following is true?
A scientist prepares an experiment to demonstrate the second law of thermodynamics for a chemistry class. In order to conduct the experiment, the scientist brings the class outside in January and gathers a cup of water and a portable stove.
The temperature outside is –10 degrees Celsius. The scientist asks the students to consider the following when answering his questions:
Gibbs Free Energy Formula:
ΔG = ΔH – TΔS
Liquid-Solid Water Phase Change Reaction:
H2O(l) ⇌ H2O(s) + X
The scientist prepares two scenarios.
Scenario 1:
The scientist buries the cup of water outside in the snow, returns to the classroom with his class for one hour, and the class then checks on the cup. They find that the water has frozen in the cup.
Scenario 2:
The scientist then places the frozen cup of water on the stove and starts the gas. The class finds that the water melts quickly.
After the water melts, the scientist asks the students to consider two hypothetical scenarios as a thought experiment.
Scenario 3:
Once the liquid water at the end of scenario 2 melts completely, the scientist turns off the gas and monitors what happens to the water. Despite being in the cold air, the water never freezes.
Scenario 4:
The scientist takes the frozen water from the end of scenario 1, puts it on the active stove, and the water remains frozen.
In this situation described in the passage, which of the following is true?
Tap to reveal answer
The Gibbs Free Energy equation makes use of clearly delineated systems and surroundings. In this example, the water is freezing or melting depending on conditions. This is accompanied by thermal exchanges with other players, such as the snow and stove. Thus, the water is the system, and everything else (technically, everything else in the universe) comprises the surroundings.
The Gibbs Free Energy equation makes use of clearly delineated systems and surroundings. In this example, the water is freezing or melting depending on conditions. This is accompanied by thermal exchanges with other players, such as the snow and stove. Thus, the water is the system, and everything else (technically, everything else in the universe) comprises the surroundings.
← Didn't Know|Knew It →
A 50g sample of an unknown substance is heated to 100oC in a tub of boiling water. It is then quickly removed and placed into an insulated jar holding 200mL of water, initially at 20oC. The final equilbrium temperature of the system is 30oC. Approximately, what is the specific heat of this unknown substance?
Specific heat of water is 4.187J/goC.
Density of water is 1g/mL.
A 50g sample of an unknown substance is heated to 100oC in a tub of boiling water. It is then quickly removed and placed into an insulated jar holding 200mL of water, initially at 20oC. The final equilbrium temperature of the system is 30oC. Approximately, what is the specific heat of this unknown substance?
Specific heat of water is 4.187J/goC.
Density of water is 1g/mL.
Tap to reveal answer
Since the system is isolated, the amount of heat transferred away from the unknown substance must equal the heat transferred to the water. To calculate these heats, use 
For the water,  .
.
For the unknown substance, 
Set these values equal to get cx = 2.39 J/goC, approximately 2.4 J/goC.
Since the system is isolated, the amount of heat transferred away from the unknown substance must equal the heat transferred to the water. To calculate these heats, use
For the water, 
For the unknown substance,
Set these values equal to get cx = 2.39 J/goC, approximately 2.4 J/goC.
← Didn't Know|Knew It →




How much energy is needed to change a 50g ice cube at -30oC into 50g of water at 40oC? Use the above quantities as needed, and round the answer to the nearest kJ.
How much energy is needed to change a 50g ice cube at -30oC into 50g of water at 40oC? Use the above quantities as needed, and round the answer to the nearest kJ.
Tap to reveal answer
There are three distinct steps in this transformation: 1) heating the ice from -30oC to 0oC, 2) melting the ice at 0oC, and 3) heating the water from 0oC to 40oC.
When there is a temperature change, we use  , and when there is melting or freezing, we use
, and when there is melting or freezing, we use  .
.
-

-

-

Adding these three pieces together gives the total enthalpy change, which is equal to change in energy.

There are three distinct steps in this transformation: 1) heating the ice from -30oC to 0oC, 2) melting the ice at 0oC, and 3) heating the water from 0oC to 40oC.
When there is a temperature change, we use 

Adding these three pieces together gives the total enthalpy change, which is equal to change in energy.
← Didn't Know|Knew It →
The combustion of propane is given by the following formula.

If the heats of formation for CO, CO2, H2O, and C3H8 are -110.5 kJ/mol, -393.5 kJ/mol, -241.8 kJ/mol, and -103.85 kJ/mol, respectively, what is the heat of reaction for the combustion of propane?
The combustion of propane is given by the following formula.
If the heats of formation for CO, CO2, H2O, and C3H8 are -110.5 kJ/mol, -393.5 kJ/mol, -241.8 kJ/mol, and -103.85 kJ/mol, respectively, what is the heat of reaction for the combustion of propane?
Tap to reveal answer
Given the fact that combustion reactions are exothermic, you should expect the heat of reaction to be negative (ruling out two answer choices). The heat of reaction is equal to the heat of formation of the products minus the heat of formation of the reactants. Be sure to refer to the balanced equation for the correct number of moles for each compound.





Oxygen is not included, as it is in elemental form and therefore has a heat of formation equal to zero.
Given the fact that combustion reactions are exothermic, you should expect the heat of reaction to be negative (ruling out two answer choices). The heat of reaction is equal to the heat of formation of the products minus the heat of formation of the reactants. Be sure to refer to the balanced equation for the correct number of moles for each compound.
Oxygen is not included, as it is in elemental form and therefore has a heat of formation equal to zero.
← Didn't Know|Knew It →
A 200g sample of gold is subjected to 1.2kJ of heat.
The specific heat capacity for gold is  .
.
What is the change in temperature as a result of the heating?
A 200g sample of gold is subjected to 1.2kJ of heat.
The specific heat capacity for gold is 
What is the change in temperature as a result of the heating?
Tap to reveal answer
When a specific heat capacity is given, we typically use the equation  . Since we know all of the factors except for the change in temperature, we can simply solve for
. Since we know all of the factors except for the change in temperature, we can simply solve for  .
.



When a specific heat capacity is given, we typically use the equation 

← Didn't Know|Knew It →
A 35g piece of aluminum at a temperature of 373K is placed into 150g of water at a temperature of 298K. The aluminum and water eventually become the same temperature. No heat is released to the surroundings.
Water has a specific heat capacity of  and aluminum has a specific heat capacity of
and aluminum has a specific heat capacity of  .
.
What is the final temperature of the water and aluminum in the container?
A 35g piece of aluminum at a temperature of 373K is placed into 150g of water at a temperature of 298K. The aluminum and water eventually become the same temperature. No heat is released to the surroundings.
Water has a specific heat capacity of 

What is the final temperature of the water and aluminum in the container?
Tap to reveal answer
In this problem, we need to track the transfer of heat from the aluminum to the water. Since the heat acquired by the water is equal to the heat given off by the aluminum, we can set their equations equal to each other; however, in order to avoid a negative number, aluminum's change in temperature will be set as the initial temperature minus the final temperature. This results in the equation below.





In this problem, we need to track the transfer of heat from the aluminum to the water. Since the heat acquired by the water is equal to the heat given off by the aluminum, we can set their equations equal to each other; however, in order to avoid a negative number, aluminum's change in temperature will be set as the initial temperature minus the final temperature. This results in the equation below.

← Didn't Know|Knew It →
The combustion of liquid hexane in air at 298K gives gaseous carbon dioxide and liquid water, as shown in this reaction.

 of
of  is
is  .
.
 of
of 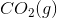 is
is .
.
 of
of 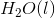 is
is  .
.
Calculate the  for the combustion of hexane liquid hexane at 298K.
for the combustion of hexane liquid hexane at 298K.
The combustion of liquid hexane in air at 298K gives gaseous carbon dioxide and liquid water, as shown in this reaction.




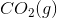


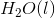

Calculate the 
Tap to reveal answer
To calculate the  , the following formula is used. Remember that the coefficients of the balanced chemical equation must be included, as shown. Also, recall that the
, the following formula is used. Remember that the coefficients of the balanced chemical equation must be included, as shown. Also, recall that the  of any pure element is zero.
of any pure element is zero.


Now we can plug in the given values and solve for the enthalpy of reaction.



To calculate the 

Now we can plug in the given values and solve for the enthalpy of reaction.
← Didn't Know|Knew It →
How much energy must a stove transfer to completely transform  of water at
of water at  into steam?
into steam?


How much energy must a stove transfer to completely transform 

Tap to reveal answer
To turn the water into steam, the stove must first raise the temperature of the water, and then provide energy to change the phase. This requires two distinct steps.
Find the energy to raise the temperature of the water using the equation  . We know our mass, the specific heat of water, and the change in temperature. Use the given values to find the necessary heat.
. We know our mass, the specific heat of water, and the change in temperature. Use the given values to find the necessary heat.


Now, we need to find the energy needed to convert the water to a gas. We will use the equation  .
.

Finally, we add the energy for the two steps to find the total energy required.

To turn the water into steam, the stove must first raise the temperature of the water, and then provide energy to change the phase. This requires two distinct steps.
Find the energy to raise the temperature of the water using the equation 
Now, we need to find the energy needed to convert the water to a gas. We will use the equation 
Finally, we add the energy for the two steps to find the total energy required.
← Didn't Know|Knew It →
Which term correctly identifies a system in which there is an exchange of heat with the surrounding environment, but no exchange of matter?
Which term correctly identifies a system in which there is an exchange of heat with the surrounding environment, but no exchange of matter?
Tap to reveal answer
In a closed system, energy and work can be exchanged with the surrounding area but matter is contained. Consider, for example, a beaker of ice. Heat from the surroundings it transferred to the ice, causing it to melt, but there is no exchange of matter. No external compounds react with the ice and none of the ice leaves the beaker.
In an open system, both heat and matter can be exchanged. In an isolated system neither heat, nor matter is exchanged.
In a closed system, energy and work can be exchanged with the surrounding area but matter is contained. Consider, for example, a beaker of ice. Heat from the surroundings it transferred to the ice, causing it to melt, but there is no exchange of matter. No external compounds react with the ice and none of the ice leaves the beaker.
In an open system, both heat and matter can be exchanged. In an isolated system neither heat, nor matter is exchanged.
← Didn't Know|Knew It →



You begin an experiment with  of ice at
of ice at  . How much heat is required to melt the ice and raise its temperature to
. How much heat is required to melt the ice and raise its temperature to  ?
?
You begin an experiment with 


Tap to reveal answer
This process can be divided into three stages: the raising of the temperature of the ice, the melting of the ice into water, and the raising of the temperature of the water. The first and third step use the specific heat of the substance to calculate the heat required, while the second step uses the heat of fusion.
Step 1 and 3: 
Step 2: 
We know the mass of the water, the change in temperature, and the constant values. Using these values, we can sum the heat required for each step to get our final answer.





This process can be divided into three stages: the raising of the temperature of the ice, the melting of the ice into water, and the raising of the temperature of the water. The first and third step use the specific heat of the substance to calculate the heat required, while the second step uses the heat of fusion.
Step 1 and 3:
Step 2:
We know the mass of the water, the change in temperature, and the constant values. Using these values, we can sum the heat required for each step to get our final answer.
← Didn't Know|Knew It →



Given the enthalpies of formation, what is the enthalpy of combustion of octane in the reaction:

Given the enthalpies of formation, what is the enthalpy of combustion of octane in the reaction:
Tap to reveal answer
The equation for enthalpy of reaction is:

Given our chemical reaction and the enthalpies of formation, we can find the enthalpy of reaction.

First, find the total enthalpy for the products.


Then, find the total enthalpy for the reactants.


Since the oxygen is elemental, its heat of formation is zero.
Return to the original equation to calculate the final enthalpy of reaction.

The equation for enthalpy of reaction is:
Given our chemical reaction and the enthalpies of formation, we can find the enthalpy of reaction.
First, find the total enthalpy for the products.
Then, find the total enthalpy for the reactants.
Since the oxygen is elemental, its heat of formation is zero.
Return to the original equation to calculate the final enthalpy of reaction.
← Didn't Know|Knew It →
Use the following values for water as needed.




If burning wood releases  of heat energy per gram of wood consumed, what mass of wood must be consumed to heat
of heat energy per gram of wood consumed, what mass of wood must be consumed to heat  of water from
of water from  to
to  , and then to convert it to water vapor?
, and then to convert it to water vapor?
Use the following values for water as needed.
If burning wood releases 



Tap to reveal answer
There are two processes requiring added heat in this problem:
1. Raising the temperature of the liquid water from  to
to  (use
(use  )
)
2. Boiling the water at a constant temperature of  (use
(use  )
)
To use either of these equation, we need to find the mass of the water using the relation between mass, density, and volume.

Use this mass with the given specific heat and temperatures to find the heat for part 1 of the process.

Then, use the mass with the given heat of vaporization to find the energy needed to convert the water to water vapor.

Sum the energies for step 1 and step 2.

This is the total amount of energy needed from the burning wood. Use stoichiometry to find the grams of wood needed to produce this amount of energy.

There are two processes requiring added heat in this problem:
1. Raising the temperature of the liquid water from 


2. Boiling the water at a constant temperature of 

To use either of these equation, we need to find the mass of the water using the relation between mass, density, and volume.
Use this mass with the given specific heat and temperatures to find the heat for part 1 of the process.
Then, use the mass with the given heat of vaporization to find the energy needed to convert the water to water vapor.
Sum the energies for step 1 and step 2.
This is the total amount of energy needed from the burning wood. Use stoichiometry to find the grams of wood needed to produce this amount of energy.
← Didn't Know|Knew It →
A metal object that is dropped into water. In which scenario would heat be transferred from the object to the water?

A metal object that is dropped into water. In which scenario would heat be transferred from the object to the water?
Tap to reveal answer
If heat is transferred from the metal object to the water, that means that the temperature of the metal must be greater than that of the water. We must find the answer choice where this is the case. Since the temperatures are given in different units, they must be converted to the same unit for comparison purposes.


Only one answer option results in the metal having a greater temperature than the water:


If heat is transferred from the metal object to the water, that means that the temperature of the metal must be greater than that of the water. We must find the answer choice where this is the case. Since the temperatures are given in different units, they must be converted to the same unit for comparison purposes.
Only one answer option results in the metal having a greater temperature than the water:
← Didn't Know|Knew It →
A scientist prepares an experiment to demonstrate the second law of thermodynamics for a chemistry class. In order to conduct the experiment, the scientist brings the class outside in January and gathers a cup of water and a portable stove.
The temperature outside is –10 degrees Celsius. The scientist asks the students to consider the following when answering his questions:
Gibbs Free Energy Formula:
ΔG = ΔH – TΔS
Liquid-Solid Water Phase Change Reaction:
H2O(l) ⇌ H2O(s) + X
The scientist prepares two scenarios.
Scenario 1:
The scientist buries the cup of water outside in the snow, returns to the classroom with his class for one hour, and the class then checks on the cup. They find that the water has frozen in the cup.
Scenario 2:
The scientist then places the frozen cup of water on the stove and starts the gas. The class finds that the water melts quickly.
After the water melts, the scientist asks the students to consider two hypothetical scenarios as a thought experiment.
Scenario 3:
Once the liquid water at the end of scenario 2 melts completely, the scientist turns off the gas and monitors what happens to the water. Despite being in the cold air, the water never freezes.
Scenario 4:
The scientist takes the frozen water from the end of scenario 1, puts it on the active stove, and the water remains frozen.
In this situation described in the passage, which of the following is true?
A scientist prepares an experiment to demonstrate the second law of thermodynamics for a chemistry class. In order to conduct the experiment, the scientist brings the class outside in January and gathers a cup of water and a portable stove.
The temperature outside is –10 degrees Celsius. The scientist asks the students to consider the following when answering his questions:
Gibbs Free Energy Formula:
ΔG = ΔH – TΔS
Liquid-Solid Water Phase Change Reaction:
H2O(l) ⇌ H2O(s) + X
The scientist prepares two scenarios.
Scenario 1:
The scientist buries the cup of water outside in the snow, returns to the classroom with his class for one hour, and the class then checks on the cup. They find that the water has frozen in the cup.
Scenario 2:
The scientist then places the frozen cup of water on the stove and starts the gas. The class finds that the water melts quickly.
After the water melts, the scientist asks the students to consider two hypothetical scenarios as a thought experiment.
Scenario 3:
Once the liquid water at the end of scenario 2 melts completely, the scientist turns off the gas and monitors what happens to the water. Despite being in the cold air, the water never freezes.
Scenario 4:
The scientist takes the frozen water from the end of scenario 1, puts it on the active stove, and the water remains frozen.
In this situation described in the passage, which of the following is true?
Tap to reveal answer
The Gibbs Free Energy equation makes use of clearly delineated systems and surroundings. In this example, the water is freezing or melting depending on conditions. This is accompanied by thermal exchanges with other players, such as the snow and stove. Thus, the water is the system, and everything else (technically, everything else in the universe) comprises the surroundings.
The Gibbs Free Energy equation makes use of clearly delineated systems and surroundings. In this example, the water is freezing or melting depending on conditions. This is accompanied by thermal exchanges with other players, such as the snow and stove. Thus, the water is the system, and everything else (technically, everything else in the universe) comprises the surroundings.
← Didn't Know|Knew It →
A 50g sample of an unknown substance is heated to 100oC in a tub of boiling water. It is then quickly removed and placed into an insulated jar holding 200mL of water, initially at 20oC. The final equilbrium temperature of the system is 30oC. Approximately, what is the specific heat of this unknown substance?
Specific heat of water is 4.187J/goC.
Density of water is 1g/mL.
A 50g sample of an unknown substance is heated to 100oC in a tub of boiling water. It is then quickly removed and placed into an insulated jar holding 200mL of water, initially at 20oC. The final equilbrium temperature of the system is 30oC. Approximately, what is the specific heat of this unknown substance?
Specific heat of water is 4.187J/goC.
Density of water is 1g/mL.
Tap to reveal answer
Since the system is isolated, the amount of heat transferred away from the unknown substance must equal the heat transferred to the water. To calculate these heats, use 
For the water,  .
.
For the unknown substance, 
Set these values equal to get cx = 2.39 J/goC, approximately 2.4 J/goC.
Since the system is isolated, the amount of heat transferred away from the unknown substance must equal the heat transferred to the water. To calculate these heats, use
For the water, 
For the unknown substance,
Set these values equal to get cx = 2.39 J/goC, approximately 2.4 J/goC.
← Didn't Know|Knew It →


In the above reaction, how much heat will be released if 74.0g of sulfur reacts with excess oxygen? Round to the nearest 10kJ.

In the above reaction, how much heat will be released if 74.0g of sulfur reacts with excess oxygen? Round to the nearest 10kJ.
Tap to reveal answer
Basically, this is a unit conversion problem. Starting with grams of sulfur, convert to moles of sulfur, and finally to kJ of heat released. Note that the given enthalpy of reaction,  , is the amount of heat released when two moles of sulfur react with three moles of oxygen.
, is the amount of heat released when two moles of sulfur react with three moles of oxygen.

Note that a negative enthalpy of reaction means the process is exothermic and releases heat, while a positive enthalpy of reaction would mean the process is endothermic and absorbs heat.
Basically, this is a unit conversion problem. Starting with grams of sulfur, convert to moles of sulfur, and finally to kJ of heat released. Note that the given enthalpy of reaction, 
Note that a negative enthalpy of reaction means the process is exothermic and releases heat, while a positive enthalpy of reaction would mean the process is endothermic and absorbs heat.
← Didn't Know|Knew It →




How much energy is needed to change a 50g ice cube at -30oC into 50g of water at 40oC? Use the above quantities as needed, and round the answer to the nearest kJ.
How much energy is needed to change a 50g ice cube at -30oC into 50g of water at 40oC? Use the above quantities as needed, and round the answer to the nearest kJ.
Tap to reveal answer
There are three distinct steps in this transformation: 1) heating the ice from -30oC to 0oC, 2) melting the ice at 0oC, and 3) heating the water from 0oC to 40oC.
When there is a temperature change, we use  , and when there is melting or freezing, we use
, and when there is melting or freezing, we use  .
.
-

-

-

Adding these three pieces together gives the total enthalpy change, which is equal to change in energy.

There are three distinct steps in this transformation: 1) heating the ice from -30oC to 0oC, 2) melting the ice at 0oC, and 3) heating the water from 0oC to 40oC.
When there is a temperature change, we use 

Adding these three pieces together gives the total enthalpy change, which is equal to change in energy.
← Didn't Know|Knew It →
The combustion of propane is given by the following formula.

If the heats of formation for CO, CO2, H2O, and C3H8 are -110.5 kJ/mol, -393.5 kJ/mol, -241.8 kJ/mol, and -103.85 kJ/mol, respectively, what is the heat of reaction for the combustion of propane?
The combustion of propane is given by the following formula.
If the heats of formation for CO, CO2, H2O, and C3H8 are -110.5 kJ/mol, -393.5 kJ/mol, -241.8 kJ/mol, and -103.85 kJ/mol, respectively, what is the heat of reaction for the combustion of propane?
Tap to reveal answer
Given the fact that combustion reactions are exothermic, you should expect the heat of reaction to be negative (ruling out two answer choices). The heat of reaction is equal to the heat of formation of the products minus the heat of formation of the reactants. Be sure to refer to the balanced equation for the correct number of moles for each compound.





Oxygen is not included, as it is in elemental form and therefore has a heat of formation equal to zero.
Given the fact that combustion reactions are exothermic, you should expect the heat of reaction to be negative (ruling out two answer choices). The heat of reaction is equal to the heat of formation of the products minus the heat of formation of the reactants. Be sure to refer to the balanced equation for the correct number of moles for each compound.
Oxygen is not included, as it is in elemental form and therefore has a heat of formation equal to zero.
← Didn't Know|Knew It →
A 200g sample of gold is subjected to 1.2kJ of heat.
The specific heat capacity for gold is  .
.
What is the change in temperature as a result of the heating?
A 200g sample of gold is subjected to 1.2kJ of heat.
The specific heat capacity for gold is 
What is the change in temperature as a result of the heating?
Tap to reveal answer
When a specific heat capacity is given, we typically use the equation  . Since we know all of the factors except for the change in temperature, we can simply solve for
. Since we know all of the factors except for the change in temperature, we can simply solve for  .
.



When a specific heat capacity is given, we typically use the equation 

← Didn't Know|Knew It →