Thermochemistry and Energetics - MCAT Chemical and Physical Foundations of Biological Systems
Card 1 of 518
A specific element is kept in two separate containers. The samples are both heated by the same amount of heat, but the element in container 1 experiences a greater temperature change than the element in container 2.
Which of the following could NOT be an explanation for this difference in temperature change?
A specific element is kept in two separate containers. The samples are both heated by the same amount of heat, but the element in container 1 experiences a greater temperature change than the element in container 2.
Which of the following could NOT be an explanation for this difference in temperature change?
Tap to reveal answer
If there was more mass (in grams) of the element in container 1 than there was in container 2, we would expect container 1 to undergo a smaller temperature change compared to container 2. This is justified by the equation  , where m is the mass.
, where m is the mass.
Rewritten as  , we can see that a larger mass would result in a smaller temperature change. Remember that q is held constant between the two containers.
, we can see that a larger mass would result in a smaller temperature change. Remember that q is held constant between the two containers.
If element 2 expelled some of its energy to the surroundings in the form of work (expanding the container), it could explain why it had a smaller temperature change. In addition, different phases of an element have different specific heat capacities. This can also explain the difference in temperature between the two element samples.
If there was more mass (in grams) of the element in container 1 than there was in container 2, we would expect container 1 to undergo a smaller temperature change compared to container 2. This is justified by the equation 
Rewritten as 
If element 2 expelled some of its energy to the surroundings in the form of work (expanding the container), it could explain why it had a smaller temperature change. In addition, different phases of an element have different specific heat capacities. This can also explain the difference in temperature between the two element samples.
← Didn't Know|Knew It →
A burning tree in a forest causes another tree that is twenty inches away to also start burning, without touching it. How is the heat being transferred from the burning tree to the other?
A burning tree in a forest causes another tree that is twenty inches away to also start burning, without touching it. How is the heat being transferred from the burning tree to the other?
Tap to reveal answer
Convection describes heat transfer through a fluid medium, such as a gas or liquid. In this case, the burning tree transfers heat to the air, which transfers the heat to the other tree.
Conduction requires direct contact, which would occur if a burning tree fell into another tree. Radiation is the electromagnetic transfer of heat, such as the sun's heat that travels to Earth, and does not require matter to transfer. Transduction is not a mechanism for heat transfer.
Convection describes heat transfer through a fluid medium, such as a gas or liquid. In this case, the burning tree transfers heat to the air, which transfers the heat to the other tree.
Conduction requires direct contact, which would occur if a burning tree fell into another tree. Radiation is the electromagnetic transfer of heat, such as the sun's heat that travels to Earth, and does not require matter to transfer. Transduction is not a mechanism for heat transfer.
← Didn't Know|Knew It →
Which of the following is not related to bond dissociation energy?
Which of the following is not related to bond dissociation energy?
Tap to reveal answer
Bond dissociation energy is the energy associated with a bond within a molecule. This means that bond dissociation energy is measured for intramolecular bonds. All covalent and ionic bonds are considered intramolecular bonds, and are generally quite permanent. Ionic bonds and polar covalent bonds can help develop dipoles in a molecule, which later facilitate intermolecular interactions.
Hydrogen bonds are intermolecular bonds, which do not have associated bond dissociation energies because these type of bonds are temporary, and are formed between different molecules.
Bond dissociation energy is the energy associated with a bond within a molecule. This means that bond dissociation energy is measured for intramolecular bonds. All covalent and ionic bonds are considered intramolecular bonds, and are generally quite permanent. Ionic bonds and polar covalent bonds can help develop dipoles in a molecule, which later facilitate intermolecular interactions.
Hydrogen bonds are intermolecular bonds, which do not have associated bond dissociation energies because these type of bonds are temporary, and are formed between different molecules.
← Didn't Know|Knew It →
A burning tree in a forest causes another tree that is twenty inches away to also start burning, without touching it. How is the heat being transferred from the burning tree to the other?
A burning tree in a forest causes another tree that is twenty inches away to also start burning, without touching it. How is the heat being transferred from the burning tree to the other?
Tap to reveal answer
Convection describes heat transfer through a fluid medium, such as a gas or liquid. In this case, the burning tree transfers heat to the air, which transfers the heat to the other tree.
Conduction requires direct contact, which would occur if a burning tree fell into another tree. Radiation is the electromagnetic transfer of heat, such as the sun's heat that travels to Earth, and does not require matter to transfer. Transduction is not a mechanism for heat transfer.
Convection describes heat transfer through a fluid medium, such as a gas or liquid. In this case, the burning tree transfers heat to the air, which transfers the heat to the other tree.
Conduction requires direct contact, which would occur if a burning tree fell into another tree. Radiation is the electromagnetic transfer of heat, such as the sun's heat that travels to Earth, and does not require matter to transfer. Transduction is not a mechanism for heat transfer.
← Didn't Know|Knew It →
Which of the following is not related to bond dissociation energy?
Which of the following is not related to bond dissociation energy?
Tap to reveal answer
Bond dissociation energy is the energy associated with a bond within a molecule. This means that bond dissociation energy is measured for intramolecular bonds. All covalent and ionic bonds are considered intramolecular bonds, and are generally quite permanent. Ionic bonds and polar covalent bonds can help develop dipoles in a molecule, which later facilitate intermolecular interactions.
Hydrogen bonds are intermolecular bonds, which do not have associated bond dissociation energies because these type of bonds are temporary, and are formed between different molecules.
Bond dissociation energy is the energy associated with a bond within a molecule. This means that bond dissociation energy is measured for intramolecular bonds. All covalent and ionic bonds are considered intramolecular bonds, and are generally quite permanent. Ionic bonds and polar covalent bonds can help develop dipoles in a molecule, which later facilitate intermolecular interactions.
Hydrogen bonds are intermolecular bonds, which do not have associated bond dissociation energies because these type of bonds are temporary, and are formed between different molecules.
← Didn't Know|Knew It →
A specific element is kept in two separate containers. The samples are both heated by the same amount of heat, but the element in container 1 experiences a greater temperature change than the element in container 2.
Which of the following could NOT be an explanation for this difference in temperature change?
A specific element is kept in two separate containers. The samples are both heated by the same amount of heat, but the element in container 1 experiences a greater temperature change than the element in container 2.
Which of the following could NOT be an explanation for this difference in temperature change?
Tap to reveal answer
If there was more mass (in grams) of the element in container 1 than there was in container 2, we would expect container 1 to undergo a smaller temperature change compared to container 2. This is justified by the equation  , where m is the mass.
, where m is the mass.
Rewritten as  , we can see that a larger mass would result in a smaller temperature change. Remember that q is held constant between the two containers.
, we can see that a larger mass would result in a smaller temperature change. Remember that q is held constant between the two containers.
If element 2 expelled some of its energy to the surroundings in the form of work (expanding the container), it could explain why it had a smaller temperature change. In addition, different phases of an element have different specific heat capacities. This can also explain the difference in temperature between the two element samples.
If there was more mass (in grams) of the element in container 1 than there was in container 2, we would expect container 1 to undergo a smaller temperature change compared to container 2. This is justified by the equation 
Rewritten as 
If element 2 expelled some of its energy to the surroundings in the form of work (expanding the container), it could explain why it had a smaller temperature change. In addition, different phases of an element have different specific heat capacities. This can also explain the difference in temperature between the two element samples.
← Didn't Know|Knew It →
Which of the following processes involves an increase in entropy?
Which of the following processes involves an increase in entropy?
Tap to reveal answer
Entropy is a measure of disorder or randomness. A system with more random motion between molecules has greater entropy. The phases of matter in order of increasing entropy are solid, liquid, then gas. The processes that increase entropy by changing phases will cause a phase transition from lower entropy to higher entropy. These transitions are melting (solid to liquid), vaporization (liquid to gas), and sublimation (solid to gas).
Entropy is a measure of disorder or randomness. A system with more random motion between molecules has greater entropy. The phases of matter in order of increasing entropy are solid, liquid, then gas. The processes that increase entropy by changing phases will cause a phase transition from lower entropy to higher entropy. These transitions are melting (solid to liquid), vaporization (liquid to gas), and sublimation (solid to gas).
← Didn't Know|Knew It →
A scientist prepares an experiment to demonstrate the second law of thermodynamics for a chemistry class. In order to conduct the experiment, the scientist brings the class outside in January and gathers a cup of water and a portable stove.
The temperature outside is –10 degrees Celsius. The scientist asks the students to consider the following when answering his questions:
Gibbs Free Energy Formula:
ΔG = ΔH – TΔS
Liquid-Solid Water Phase Change Reaction:
H2O(l) ⇌ H2O(s) + X
The scientist prepares two scenarios.
Scenario 1:
The scientist buries the cup of water outside in the snow, returns to the classroom with his class for one hour, and the class then checks on the cup. They find that the water has frozen in the cup.
Scenario 2:
The scientist then places the frozen cup of water on the stove and starts the gas. The class finds that the water melts quickly.
After the water melts, the scientist asks the students to consider two hypothetical scenarios as a thought experiment.
Scenario 3:
Once the liquid water at the end of scenario 2 melts completely, the scientist turns off the gas and monitors what happens to the water. Despite being in the cold air, the water never freezes.
Scenario 4:
The scientist takes the frozen water from the end of scenario 1, puts it on the active stove, and the water remains frozen.
After the processes in each of the four scenarios above, what can be said about the entropy of the universe?
A scientist prepares an experiment to demonstrate the second law of thermodynamics for a chemistry class. In order to conduct the experiment, the scientist brings the class outside in January and gathers a cup of water and a portable stove.
The temperature outside is –10 degrees Celsius. The scientist asks the students to consider the following when answering his questions:
Gibbs Free Energy Formula:
ΔG = ΔH – TΔS
Liquid-Solid Water Phase Change Reaction:
H2O(l) ⇌ H2O(s) + X
The scientist prepares two scenarios.
Scenario 1:
The scientist buries the cup of water outside in the snow, returns to the classroom with his class for one hour, and the class then checks on the cup. They find that the water has frozen in the cup.
Scenario 2:
The scientist then places the frozen cup of water on the stove and starts the gas. The class finds that the water melts quickly.
After the water melts, the scientist asks the students to consider two hypothetical scenarios as a thought experiment.
Scenario 3:
Once the liquid water at the end of scenario 2 melts completely, the scientist turns off the gas and monitors what happens to the water. Despite being in the cold air, the water never freezes.
Scenario 4:
The scientist takes the frozen water from the end of scenario 1, puts it on the active stove, and the water remains frozen.
After the processes in each of the four scenarios above, what can be said about the entropy of the universe?
Tap to reveal answer
Scenarios 3 and 4 are presented as thought experiments because, common sense tells us, they will never happen. The fundamental reason they will never happen is because they do not lead to a total increase in entropy of the universe. In order for an event to proceed, this requirement must be satisfied.
Scenarios 3 and 4 are presented as thought experiments because, common sense tells us, they will never happen. The fundamental reason they will never happen is because they do not lead to a total increase in entropy of the universe. In order for an event to proceed, this requirement must be satisfied.
← Didn't Know|Knew It →
Which of the following chemical or physical processes involves an increase in entropy?
Which of the following chemical or physical processes involves an increase in entropy?
Tap to reveal answer
Entropy describes the number of configurations a system can have or, alternatively, how much "order" it possesses.
When water freezes, it forms a crystal lattice, which is more ordered than liquid water. Likewise, collecting playing cards and stacking them in a deck involves a transformation from disorder (cards scattered around the floor) to order (a single deck). Chemically, the precipitation of  from aqueous ions comes from a decrease in entropy, as the free-flowing ions have been combined to form a solid, organized in a lattice. The combination of
from aqueous ions comes from a decrease in entropy, as the free-flowing ions have been combined to form a solid, organized in a lattice. The combination of  and
and  to give
to give  also results in a decrease in entropy, since the reactants have one mole of gas, and the products contain zero moles of gas.
also results in a decrease in entropy, since the reactants have one mole of gas, and the products contain zero moles of gas.
The disproportionation of  to give two moles of
to give two moles of  results in an increase in entropy, because the number of moles of gas increases in going from reactants to products.
results in an increase in entropy, because the number of moles of gas increases in going from reactants to products.
Entropy describes the number of configurations a system can have or, alternatively, how much "order" it possesses.
When water freezes, it forms a crystal lattice, which is more ordered than liquid water. Likewise, collecting playing cards and stacking them in a deck involves a transformation from disorder (cards scattered around the floor) to order (a single deck). Chemically, the precipitation of 



The disproportionation of 

← Didn't Know|Knew It →
Acids and bases can be described in three principal ways. The Arrhenius definition is the most restrictive. It limits acids and bases to species that donate protons and hydroxide ions in solution, respectively. Examples of such acids include HCl and HBr, while KOH and NaOH are examples of bases. When in aqueous solution, these acids proceed to an equilibrium state through a dissociation reaction.

All of the bases proceed in a similar fashion.

The Brønsted-Lowry definition of an acid is a more inclusive approach. All Arrhenius acids and bases are also Brønsted-Lowry acids and bases, but the converse is not true. Brønsted-Lowry acids still reach equilibrium through the same dissociation reaction as Arrhenius acids, but the acid character is defined by different parameters. The Brønsted-Lowry definition considers bases to be hydroxide donors, like the Arrhenius definition, but also includes conjugate bases such as the A- in the above reaction. In the reverse reaction, A- accepts the proton to regenerate HA. The Brønsted-Lowry definition thus defines bases as proton acceptors, and acids as proton donors.
A scientist studying the dissociation of the Arrhenius base sodium hydroxide discovers that the reaction is very exothermic. What is true of the entropy change in the surroundings?
Assume the system refers to the reaction vesselcontaining aqueous sodium hydroxide.
Acids and bases can be described in three principal ways. The Arrhenius definition is the most restrictive. It limits acids and bases to species that donate protons and hydroxide ions in solution, respectively. Examples of such acids include HCl and HBr, while KOH and NaOH are examples of bases. When in aqueous solution, these acids proceed to an equilibrium state through a dissociation reaction.
All of the bases proceed in a similar fashion.
The Brønsted-Lowry definition of an acid is a more inclusive approach. All Arrhenius acids and bases are also Brønsted-Lowry acids and bases, but the converse is not true. Brønsted-Lowry acids still reach equilibrium through the same dissociation reaction as Arrhenius acids, but the acid character is defined by different parameters. The Brønsted-Lowry definition considers bases to be hydroxide donors, like the Arrhenius definition, but also includes conjugate bases such as the A- in the above reaction. In the reverse reaction, A- accepts the proton to regenerate HA. The Brønsted-Lowry definition thus defines bases as proton acceptors, and acids as proton donors.
A scientist studying the dissociation of the Arrhenius base sodium hydroxide discovers that the reaction is very exothermic. What is true of the entropy change in the surroundings?
Assume the system refers to the reaction vesselcontaining aqueous sodium hydroxide.
Tap to reveal answer
The entropy change of the surroundings for an exothermic system must be positive. The release of heat by the exothermic reaction drives the increase in disorder of the environment surrounding the reaction vessel.

As the heat leaves the reaction system, it increases the entropy of the particles in the surroundings.
The entropy change of the surroundings for an exothermic system must be positive. The release of heat by the exothermic reaction drives the increase in disorder of the environment surrounding the reaction vessel.
As the heat leaves the reaction system, it increases the entropy of the particles in the surroundings.
← Didn't Know|Knew It →
Which of the following chemical processes involves a decrease in entropy?
Which of the following chemical processes involves a decrease in entropy?
Tap to reveal answer
Entropy decreases when the number of moles of gas decreases during a reaction. In the case of the correct answer, the number of moles of gas decreases from two to one.
When a substance goes from a solid to a gas (sublimation) or from a liquid to a gas (evaporation), entropy increases. Likewise, when a solid dissolves in water, entropy increases. Entropy (i.e. the number of arrangements a system can have) is much greater in a gas than in a liquid or solid. It is also greater for ions solvated in solution than for an un-dissolved solid.
Note that, in general, the entropy of the universe increases. In order for a process to involve a decrease in entropy of the system, there is likely a consequent increase in entropy of the universe.
Entropy decreases when the number of moles of gas decreases during a reaction. In the case of the correct answer, the number of moles of gas decreases from two to one.
When a substance goes from a solid to a gas (sublimation) or from a liquid to a gas (evaporation), entropy increases. Likewise, when a solid dissolves in water, entropy increases. Entropy (i.e. the number of arrangements a system can have) is much greater in a gas than in a liquid or solid. It is also greater for ions solvated in solution than for an un-dissolved solid.
Note that, in general, the entropy of the universe increases. In order for a process to involve a decrease in entropy of the system, there is likely a consequent increase in entropy of the universe.
← Didn't Know|Knew It →
Which of the following does not represent an increase in entropy?
Which of the following does not represent an increase in entropy?
Tap to reveal answer
An increase in entropy will result when a solid is dissolved into a liquid because disorder is increased due to the presence of a greater number of particulates (ions). Similarly, any reaction that results in more molecules represents an increase in entropy, as in the example of the decomposition of hydrogen peroxide. A temperature increase does not relate to entropy; it only helps describe the energy of the reaction. An increase in temperature usually signifies an endothermic process, but does not give an indication about entropy.
The only correct answer is the chemical reaction between aluminum and iron (III) oxide, which produces an equal amount of particles and does not involve any phase change.
An increase in entropy will result when a solid is dissolved into a liquid because disorder is increased due to the presence of a greater number of particulates (ions). Similarly, any reaction that results in more molecules represents an increase in entropy, as in the example of the decomposition of hydrogen peroxide. A temperature increase does not relate to entropy; it only helps describe the energy of the reaction. An increase in temperature usually signifies an endothermic process, but does not give an indication about entropy.
The only correct answer is the chemical reaction between aluminum and iron (III) oxide, which produces an equal amount of particles and does not involve any phase change.
← Didn't Know|Knew It →


In the above reaction, how much heat will be released if 74.0g of sulfur reacts with excess oxygen? Round to the nearest 10kJ.

In the above reaction, how much heat will be released if 74.0g of sulfur reacts with excess oxygen? Round to the nearest 10kJ.
Tap to reveal answer
Basically, this is a unit conversion problem. Starting with grams of sulfur, convert to moles of sulfur, and finally to kJ of heat released. Note that the given enthalpy of reaction,  , is the amount of heat released when two moles of sulfur react with three moles of oxygen.
, is the amount of heat released when two moles of sulfur react with three moles of oxygen.

Note that a negative enthalpy of reaction means the process is exothermic and releases heat, while a positive enthalpy of reaction would mean the process is endothermic and absorbs heat.
Basically, this is a unit conversion problem. Starting with grams of sulfur, convert to moles of sulfur, and finally to kJ of heat released. Note that the given enthalpy of reaction, 
Note that a negative enthalpy of reaction means the process is exothermic and releases heat, while a positive enthalpy of reaction would mean the process is endothermic and absorbs heat.
← Didn't Know|Knew It →
A scientist prepares an experiment to demonstrate the second law of thermodynamics for a chemistry class. In order to conduct the experiment, the scientist brings the class outside in January and gathers a cup of water and a portable stove.
The temperature outside is –10 degrees Celsius. The scientist asks the students to consider the following when answering his questions:
Gibbs Free Energy Formula:
ΔG = ΔH – TΔS
Liquid-Solid Water Phase Change Reaction:
H2O(l) ⇌ H2O(s) + X
The scientist prepares two scenarios.
Scenario 1:
The scientist buries the cup of water outside in the snow, returns to the classroom with his class for one hour, and the class then checks on the cup. They find that the water has frozen in the cup.
Scenario 2:
The scientist then places the frozen cup of water on the stove and starts the gas. The class finds that the water melts quickly.
After the water melts, the scientist asks the students to consider two hypothetical scenarios as a thought experiment.
Scenario 3:
Once the liquid water at the end of scenario 2 melts completely, the scientist turns off the gas and monitors what happens to the water. Despite being in the cold air, the water never freezes.
Scenario 4:
The scientist takes the frozen water from the end of scenario 1, puts it on the active stove, and the water remains frozen.
In this situation described in the passage, which of the following is true?
A scientist prepares an experiment to demonstrate the second law of thermodynamics for a chemistry class. In order to conduct the experiment, the scientist brings the class outside in January and gathers a cup of water and a portable stove.
The temperature outside is –10 degrees Celsius. The scientist asks the students to consider the following when answering his questions:
Gibbs Free Energy Formula:
ΔG = ΔH – TΔS
Liquid-Solid Water Phase Change Reaction:
H2O(l) ⇌ H2O(s) + X
The scientist prepares two scenarios.
Scenario 1:
The scientist buries the cup of water outside in the snow, returns to the classroom with his class for one hour, and the class then checks on the cup. They find that the water has frozen in the cup.
Scenario 2:
The scientist then places the frozen cup of water on the stove and starts the gas. The class finds that the water melts quickly.
After the water melts, the scientist asks the students to consider two hypothetical scenarios as a thought experiment.
Scenario 3:
Once the liquid water at the end of scenario 2 melts completely, the scientist turns off the gas and monitors what happens to the water. Despite being in the cold air, the water never freezes.
Scenario 4:
The scientist takes the frozen water from the end of scenario 1, puts it on the active stove, and the water remains frozen.
In this situation described in the passage, which of the following is true?
Tap to reveal answer
The Gibbs Free Energy equation makes use of clearly delineated systems and surroundings. In this example, the water is freezing or melting depending on conditions. This is accompanied by thermal exchanges with other players, such as the snow and stove. Thus, the water is the system, and everything else (technically, everything else in the universe) comprises the surroundings.
The Gibbs Free Energy equation makes use of clearly delineated systems and surroundings. In this example, the water is freezing or melting depending on conditions. This is accompanied by thermal exchanges with other players, such as the snow and stove. Thus, the water is the system, and everything else (technically, everything else in the universe) comprises the surroundings.
← Didn't Know|Knew It →
A 50g sample of an unknown substance is heated to 100oC in a tub of boiling water. It is then quickly removed and placed into an insulated jar holding 200mL of water, initially at 20oC. The final equilbrium temperature of the system is 30oC. Approximately, what is the specific heat of this unknown substance?
Specific heat of water is 4.187J/goC.
Density of water is 1g/mL.
A 50g sample of an unknown substance is heated to 100oC in a tub of boiling water. It is then quickly removed and placed into an insulated jar holding 200mL of water, initially at 20oC. The final equilbrium temperature of the system is 30oC. Approximately, what is the specific heat of this unknown substance?
Specific heat of water is 4.187J/goC.
Density of water is 1g/mL.
Tap to reveal answer
Since the system is isolated, the amount of heat transferred away from the unknown substance must equal the heat transferred to the water. To calculate these heats, use 
For the water,  .
.
For the unknown substance, 
Set these values equal to get cx = 2.39 J/goC, approximately 2.4 J/goC.
Since the system is isolated, the amount of heat transferred away from the unknown substance must equal the heat transferred to the water. To calculate these heats, use
For the water, 
For the unknown substance,
Set these values equal to get cx = 2.39 J/goC, approximately 2.4 J/goC.
← Didn't Know|Knew It →




How much energy is needed to change a 50g ice cube at -30oC into 50g of water at 40oC? Use the above quantities as needed, and round the answer to the nearest kJ.
How much energy is needed to change a 50g ice cube at -30oC into 50g of water at 40oC? Use the above quantities as needed, and round the answer to the nearest kJ.
Tap to reveal answer
There are three distinct steps in this transformation: 1) heating the ice from -30oC to 0oC, 2) melting the ice at 0oC, and 3) heating the water from 0oC to 40oC.
When there is a temperature change, we use  , and when there is melting or freezing, we use
, and when there is melting or freezing, we use  .
.
-

-

-

Adding these three pieces together gives the total enthalpy change, which is equal to change in energy.

There are three distinct steps in this transformation: 1) heating the ice from -30oC to 0oC, 2) melting the ice at 0oC, and 3) heating the water from 0oC to 40oC.
When there is a temperature change, we use 

Adding these three pieces together gives the total enthalpy change, which is equal to change in energy.
← Didn't Know|Knew It →
The combustion of propane is given by the following formula.

If the heats of formation for CO, CO2, H2O, and C3H8 are -110.5 kJ/mol, -393.5 kJ/mol, -241.8 kJ/mol, and -103.85 kJ/mol, respectively, what is the heat of reaction for the combustion of propane?
The combustion of propane is given by the following formula.
If the heats of formation for CO, CO2, H2O, and C3H8 are -110.5 kJ/mol, -393.5 kJ/mol, -241.8 kJ/mol, and -103.85 kJ/mol, respectively, what is the heat of reaction for the combustion of propane?
Tap to reveal answer
Given the fact that combustion reactions are exothermic, you should expect the heat of reaction to be negative (ruling out two answer choices). The heat of reaction is equal to the heat of formation of the products minus the heat of formation of the reactants. Be sure to refer to the balanced equation for the correct number of moles for each compound.





Oxygen is not included, as it is in elemental form and therefore has a heat of formation equal to zero.
Given the fact that combustion reactions are exothermic, you should expect the heat of reaction to be negative (ruling out two answer choices). The heat of reaction is equal to the heat of formation of the products minus the heat of formation of the reactants. Be sure to refer to the balanced equation for the correct number of moles for each compound.
Oxygen is not included, as it is in elemental form and therefore has a heat of formation equal to zero.
← Didn't Know|Knew It →
A 200g sample of gold is subjected to 1.2kJ of heat.
The specific heat capacity for gold is  .
.
What is the change in temperature as a result of the heating?
A 200g sample of gold is subjected to 1.2kJ of heat.
The specific heat capacity for gold is 
What is the change in temperature as a result of the heating?
Tap to reveal answer
When a specific heat capacity is given, we typically use the equation  . Since we know all of the factors except for the change in temperature, we can simply solve for
. Since we know all of the factors except for the change in temperature, we can simply solve for  .
.



When a specific heat capacity is given, we typically use the equation 

← Didn't Know|Knew It →
A 35g piece of aluminum at a temperature of 373K is placed into 150g of water at a temperature of 298K. The aluminum and water eventually become the same temperature. No heat is released to the surroundings.
Water has a specific heat capacity of  and aluminum has a specific heat capacity of
and aluminum has a specific heat capacity of  .
.
What is the final temperature of the water and aluminum in the container?
A 35g piece of aluminum at a temperature of 373K is placed into 150g of water at a temperature of 298K. The aluminum and water eventually become the same temperature. No heat is released to the surroundings.
Water has a specific heat capacity of 

What is the final temperature of the water and aluminum in the container?
Tap to reveal answer
In this problem, we need to track the transfer of heat from the aluminum to the water. Since the heat acquired by the water is equal to the heat given off by the aluminum, we can set their equations equal to each other; however, in order to avoid a negative number, aluminum's change in temperature will be set as the initial temperature minus the final temperature. This results in the equation below.





In this problem, we need to track the transfer of heat from the aluminum to the water. Since the heat acquired by the water is equal to the heat given off by the aluminum, we can set their equations equal to each other; however, in order to avoid a negative number, aluminum's change in temperature will be set as the initial temperature minus the final temperature. This results in the equation below.

← Didn't Know|Knew It →
The combustion of liquid hexane in air at 298K gives gaseous carbon dioxide and liquid water, as shown in this reaction.

 of
of  is
is  .
.
 of
of 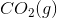 is
is .
.
 of
of 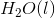 is
is  .
.
Calculate the  for the combustion of hexane liquid hexane at 298K.
for the combustion of hexane liquid hexane at 298K.
The combustion of liquid hexane in air at 298K gives gaseous carbon dioxide and liquid water, as shown in this reaction.




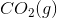


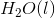

Calculate the 
Tap to reveal answer
To calculate the  , the following formula is used. Remember that the coefficients of the balanced chemical equation must be included, as shown. Also, recall that the
, the following formula is used. Remember that the coefficients of the balanced chemical equation must be included, as shown. Also, recall that the  of any pure element is zero.
of any pure element is zero.


Now we can plug in the given values and solve for the enthalpy of reaction.



To calculate the 

Now we can plug in the given values and solve for the enthalpy of reaction.
← Didn't Know|Knew It →