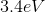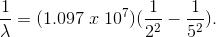Atomic Spectra
Help Questions
GRE Subject Test: Physics › Atomic Spectra
What is the energy of the photon emitted when a Hydrogen atom makes a transition from the 

Explanation


However, since the question asked what is the energy of the photon, it is the absolute value of the energy calculated above because of conservation of energy. The photon carries away the energy lost by the atom.
What color will be emitted in the 

Violet
Red
Yellow
Blue
Green
Explanation

Here, 




By inverting,