Atomic Spectra - GRE Subject Test: Physics
Card 1 of 8
What color will be emitted in the  to
to  transition in the Hydrogen Balmer series?
transition in the Hydrogen Balmer series?
What color will be emitted in the 

Tap to reveal answer
 .
.
Here,  is the wavelength,
is the wavelength,  is the Rydberg constant,
is the Rydberg constant,  for the Balmer series, and
for the Balmer series, and  refers to the upper atomic level (this is
refers to the upper atomic level (this is  in this case). Now, we just need to plug everything in, and solve for wavelength.
in this case). Now, we just need to plug everything in, and solve for wavelength.
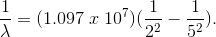


By inverting,  This is in the violet regime of the visible spectrum.
This is in the violet regime of the visible spectrum.

Here, 




By inverting, 
← Didn't Know|Knew It →
What is the energy of the photon emitted when a Hydrogen atom makes a transition from the  to the
to the  atomic energy level?
atomic energy level?
What is the energy of the photon emitted when a Hydrogen atom makes a transition from the 

Tap to reveal answer
 , where
, where  refers to the final and initial energy levels, respectively.
refers to the final and initial energy levels, respectively.

However, since the question asked what is the energy of the photon, it is the absolute value of the energy calculated above because of conservation of energy. The photon carries away the energy lost by the atom.


However, since the question asked what is the energy of the photon, it is the absolute value of the energy calculated above because of conservation of energy. The photon carries away the energy lost by the atom.
← Didn't Know|Knew It →
What color will be emitted in the  to
to  transition in the Hydrogen Balmer series?
transition in the Hydrogen Balmer series?
What color will be emitted in the 

Tap to reveal answer
 .
.
Here,  is the wavelength,
is the wavelength,  is the Rydberg constant,
is the Rydberg constant,  for the Balmer series, and
for the Balmer series, and  refers to the upper atomic level (this is
refers to the upper atomic level (this is  in this case). Now, we just need to plug everything in, and solve for wavelength.
in this case). Now, we just need to plug everything in, and solve for wavelength.
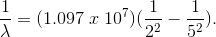


By inverting,  This is in the violet regime of the visible spectrum.
This is in the violet regime of the visible spectrum.

Here, 




By inverting, 
← Didn't Know|Knew It →
What is the energy of the photon emitted when a Hydrogen atom makes a transition from the  to the
to the  atomic energy level?
atomic energy level?
What is the energy of the photon emitted when a Hydrogen atom makes a transition from the 

Tap to reveal answer
 , where
, where  refers to the final and initial energy levels, respectively.
refers to the final and initial energy levels, respectively.

However, since the question asked what is the energy of the photon, it is the absolute value of the energy calculated above because of conservation of energy. The photon carries away the energy lost by the atom.


However, since the question asked what is the energy of the photon, it is the absolute value of the energy calculated above because of conservation of energy. The photon carries away the energy lost by the atom.
← Didn't Know|Knew It →
What color will be emitted in the  to
to  transition in the Hydrogen Balmer series?
transition in the Hydrogen Balmer series?
What color will be emitted in the 

Tap to reveal answer
 .
.
Here,  is the wavelength,
is the wavelength,  is the Rydberg constant,
is the Rydberg constant,  for the Balmer series, and
for the Balmer series, and  refers to the upper atomic level (this is
refers to the upper atomic level (this is  in this case). Now, we just need to plug everything in, and solve for wavelength.
in this case). Now, we just need to plug everything in, and solve for wavelength.
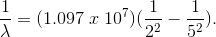


By inverting,  This is in the violet regime of the visible spectrum.
This is in the violet regime of the visible spectrum.

Here, 




By inverting, 
← Didn't Know|Knew It →
What is the energy of the photon emitted when a Hydrogen atom makes a transition from the  to the
to the  atomic energy level?
atomic energy level?
What is the energy of the photon emitted when a Hydrogen atom makes a transition from the 

Tap to reveal answer
 , where
, where  refers to the final and initial energy levels, respectively.
refers to the final and initial energy levels, respectively.

However, since the question asked what is the energy of the photon, it is the absolute value of the energy calculated above because of conservation of energy. The photon carries away the energy lost by the atom.


However, since the question asked what is the energy of the photon, it is the absolute value of the energy calculated above because of conservation of energy. The photon carries away the energy lost by the atom.
← Didn't Know|Knew It →
What color will be emitted in the  to
to  transition in the Hydrogen Balmer series?
transition in the Hydrogen Balmer series?
What color will be emitted in the 

Tap to reveal answer
 .
.
Here,  is the wavelength,
is the wavelength,  is the Rydberg constant,
is the Rydberg constant,  for the Balmer series, and
for the Balmer series, and  refers to the upper atomic level (this is
refers to the upper atomic level (this is  in this case). Now, we just need to plug everything in, and solve for wavelength.
in this case). Now, we just need to plug everything in, and solve for wavelength.
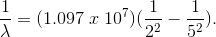


By inverting,  This is in the violet regime of the visible spectrum.
This is in the violet regime of the visible spectrum.

Here, 




By inverting, 
← Didn't Know|Knew It →
What is the energy of the photon emitted when a Hydrogen atom makes a transition from the  to the
to the  atomic energy level?
atomic energy level?
What is the energy of the photon emitted when a Hydrogen atom makes a transition from the 

Tap to reveal answer
 , where
, where  refers to the final and initial energy levels, respectively.
refers to the final and initial energy levels, respectively.

However, since the question asked what is the energy of the photon, it is the absolute value of the energy calculated above because of conservation of energy. The photon carries away the energy lost by the atom.


However, since the question asked what is the energy of the photon, it is the absolute value of the energy calculated above because of conservation of energy. The photon carries away the energy lost by the atom.
← Didn't Know|Knew It →