Protein Functions - Biochemistry
Card 1 of 116
Phosphoglucomutase is an enzyme seen in glycogen breakdown. It is responsible for converting glucose-1-phosphate ( ) to glucose-6-phosphate (
) to glucose-6-phosphate (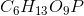 ).
).
Based on this action, to which enzyme class does phosphoglucomutase belong?
Phosphoglucomutase is an enzyme seen in glycogen breakdown. It is responsible for converting glucose-1-phosphate (
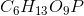
Based on this action, to which enzyme class does phosphoglucomutase belong?
Tap to reveal answer
Phosphoglucomutase is responsible for altering the position of the phosphate on the glucose from the "1" position to the "6" position. However, notice how the molecular formula for the product and the substrate are the same. Enzymes that rearrange the structure of a molecule in this manner are referred to as isomerase enzymes.
Phosphoglucomutase is responsible for altering the position of the phosphate on the glucose from the "1" position to the "6" position. However, notice how the molecular formula for the product and the substrate are the same. Enzymes that rearrange the structure of a molecule in this manner are referred to as isomerase enzymes.
← Didn't Know|Knew It →
Phosphoglucomutase is an enzyme seen in glycogen breakdown. It is responsible for converting glucose-1-phosphate ( ) to glucose-6-phosphate (
) to glucose-6-phosphate (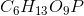 ).
).
Based on this action, to which enzyme class does phosphoglucomutase belong?
Phosphoglucomutase is an enzyme seen in glycogen breakdown. It is responsible for converting glucose-1-phosphate (
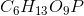
Based on this action, to which enzyme class does phosphoglucomutase belong?
Tap to reveal answer
Phosphoglucomutase is responsible for altering the position of the phosphate on the glucose from the "1" position to the "6" position. However, notice how the molecular formula for the product and the substrate are the same. Enzymes that rearrange the structure of a molecule in this manner are referred to as isomerase enzymes.
Phosphoglucomutase is responsible for altering the position of the phosphate on the glucose from the "1" position to the "6" position. However, notice how the molecular formula for the product and the substrate are the same. Enzymes that rearrange the structure of a molecule in this manner are referred to as isomerase enzymes.
← Didn't Know|Knew It →
Which of the following correctly mentions the function of a common eukaryotic ligase?
Which of the following correctly mentions the function of a common eukaryotic ligase?
Tap to reveal answer
Mammalian DNA ligase I has this function, and there are other DNA ligases which perform it in other animals and eukaryotes (prokaryotes also have their own DNA ligases). All the other functions mentioned are done by other classes of enzymes, not ligases (i.e. hydrolases, aminotransferases, oxidoreductases, etc.).
Mammalian DNA ligase I has this function, and there are other DNA ligases which perform it in other animals and eukaryotes (prokaryotes also have their own DNA ligases). All the other functions mentioned are done by other classes of enzymes, not ligases (i.e. hydrolases, aminotransferases, oxidoreductases, etc.).
← Didn't Know|Knew It →
How does an increase in carbon dioxide affect oxygen transport in hemoglobin?
How does an increase in carbon dioxide affect oxygen transport in hemoglobin?
Tap to reveal answer
A high carbon dioxide concentration will decrease the pH and produce the Bohr effect. These conditions will cause a slight conformational change in hemoglobin that results in a lower oxygen binding affinity. However, since the partial pressure of oxygen in the lungs in so high, most of the available oxygen will be loaded on to the hemoglobin anyway. Since the oxygen affinity is lowered, the hemoglobin will release the oxygen more freely, resulting in a greater oxygen to load in tissue. Functioning hemoglobin always has four cooperative subunits.
A high carbon dioxide concentration will decrease the pH and produce the Bohr effect. These conditions will cause a slight conformational change in hemoglobin that results in a lower oxygen binding affinity. However, since the partial pressure of oxygen in the lungs in so high, most of the available oxygen will be loaded on to the hemoglobin anyway. Since the oxygen affinity is lowered, the hemoglobin will release the oxygen more freely, resulting in a greater oxygen to load in tissue. Functioning hemoglobin always has four cooperative subunits.
← Didn't Know|Knew It →
Which of the following steps list in chronological order, the creation of a transport vesicle from a membrane via clathrin coating?
Which of the following steps list in chronological order, the creation of a transport vesicle from a membrane via clathrin coating?
Tap to reveal answer
The process of creating transport vesicles via clathrin coats proceeds in distinct steps. Before any budding occurs of the membrane, clathrin attaches to it, bound to adaptin which is attached to a transmembranal cargo receptor. (Cargo molecules are what trigger the creation of the vesicle in the first place.) The clathrin coating is believed to cause the membrane to bud. After the vesicle forms, dynamin uses GTP to pinch the vesicle off, and it is only then that the clathrin coat disassembles and we have a transport vesicle.
The process of creating transport vesicles via clathrin coats proceeds in distinct steps. Before any budding occurs of the membrane, clathrin attaches to it, bound to adaptin which is attached to a transmembranal cargo receptor. (Cargo molecules are what trigger the creation of the vesicle in the first place.) The clathrin coating is believed to cause the membrane to bud. After the vesicle forms, dynamin uses GTP to pinch the vesicle off, and it is only then that the clathrin coat disassembles and we have a transport vesicle.
← Didn't Know|Knew It →
Which of the following lists these molecules in order of increasing permeability to a lipid bilayer?
Which of the following lists these molecules in order of increasing permeability to a lipid bilayer?
Tap to reveal answer
Charged molecules do not permeate the lipid bilayer easily at all. So despite its small size, among our choices, a sodium ion passes least easily through. Polar molecules also have a hard (but less difficult) time passing through, and the larger the molecule, the harder that becomes, so after the sodium ion comes glucose, followed by water, which is polar but much smaller. Small, hydrophobic molecules -- such as carbon dioxide -- diffuse through most easily, because they can pass through the longest (hydrophobic) part of the membrane.
Charged molecules do not permeate the lipid bilayer easily at all. So despite its small size, among our choices, a sodium ion passes least easily through. Polar molecules also have a hard (but less difficult) time passing through, and the larger the molecule, the harder that becomes, so after the sodium ion comes glucose, followed by water, which is polar but much smaller. Small, hydrophobic molecules -- such as carbon dioxide -- diffuse through most easily, because they can pass through the longest (hydrophobic) part of the membrane.
← Didn't Know|Knew It →
Which type of transport does not require a protein?
Which type of transport does not require a protein?
Tap to reveal answer
There are two types of transport: passive transport and active transport. Active transport requires an expenditure of energy and a protein pump. Passive transport includes simple diffusion and facilitated diffusion. Diffusion is the movement of a substance from an area of high concentration to one of low concentration. Simple diffusion is the name for the diffusion process which does not require a protein; facilitated diffusion is the name for the diffusion process which requires a carrier protein for transport. Osmosis is the simple diffusion of water and thus does not require protein.
There are two types of transport: passive transport and active transport. Active transport requires an expenditure of energy and a protein pump. Passive transport includes simple diffusion and facilitated diffusion. Diffusion is the movement of a substance from an area of high concentration to one of low concentration. Simple diffusion is the name for the diffusion process which does not require a protein; facilitated diffusion is the name for the diffusion process which requires a carrier protein for transport. Osmosis is the simple diffusion of water and thus does not require protein.
← Didn't Know|Knew It →
Which of the following amino acids coordinates the heme ring in hemoglobin?
Which of the following amino acids coordinates the heme ring in hemoglobin?
Tap to reveal answer
In hemoglobin, the nitrogen group on the amino acid Histidine coordinates the heme ring in hemoglobin by binding to an iron atom located in the middle of the heme ring.
In hemoglobin, the nitrogen group on the amino acid Histidine coordinates the heme ring in hemoglobin by binding to an iron atom located in the middle of the heme ring.
← Didn't Know|Knew It →
A nonspecific hexagonal structure that functions to allow movement of molecules between adjacent cells is termed a(n) .
A nonspecific hexagonal structure that functions to allow movement of molecules between adjacent cells is termed a(n) .
Tap to reveal answer
A gap junction is a hexagonal protein that openly connects two adjacent cells. It is nonspecific, meaning it allows various different molecules and ions to travel through it. This type of transport is important for rapid communication between adjacent cells. For instance, gap junctions serve an important function in the heart - allowing the many cells present there to act as a functional syncytium. Integrins and tight junctions do not allow the passage of molecules between adjacent cells.
A gap junction is a hexagonal protein that openly connects two adjacent cells. It is nonspecific, meaning it allows various different molecules and ions to travel through it. This type of transport is important for rapid communication between adjacent cells. For instance, gap junctions serve an important function in the heart - allowing the many cells present there to act as a functional syncytium. Integrins and tight junctions do not allow the passage of molecules between adjacent cells.
← Didn't Know|Knew It →
GLUT (glucose transporters) proteins transport glucose from the blood to cells. Which of the following statements about them are true?
I. GLUT proteins are integral membrane proteins.
II . GLUT proteins have their amino and carboxyl termini on the extracellular side of the plasma membrane.
III. Binding of glucose to the transporter protein leads to a conformation change and the transport of glucose to the inner side of the membrane.
IV. GLUT-1, GLUT-3, and GLUT-4 proteins are ubiquitous; GLUT-2 is found in the liver, pancreas and kidney.
GLUT (glucose transporters) proteins transport glucose from the blood to cells. Which of the following statements about them are true?
I. GLUT proteins are integral membrane proteins.
II . GLUT proteins have their amino and carboxyl termini on the extracellular side of the plasma membrane.
III. Binding of glucose to the transporter protein leads to a conformation change and the transport of glucose to the inner side of the membrane.
IV. GLUT-1, GLUT-3, and GLUT-4 proteins are ubiquitous; GLUT-2 is found in the liver, pancreas and kidney.
Tap to reveal answer
GLUT are integral membrane proteins.The proteins cross the membrane having the amino and carboxyl termini on the cytoplasmic side of the plasma membrane. Binding of glucose to the transporter leads to a conformational change, transport of glucose to the other side of the membrane. GLUT-1, GLUT -3 and GLUT-4 are present in most tissues; GLUT-2 is found in the liver, pancreas and kidney.
GLUT are integral membrane proteins.The proteins cross the membrane having the amino and carboxyl termini on the cytoplasmic side of the plasma membrane. Binding of glucose to the transporter leads to a conformational change, transport of glucose to the other side of the membrane. GLUT-1, GLUT -3 and GLUT-4 are present in most tissues; GLUT-2 is found in the liver, pancreas and kidney.
← Didn't Know|Knew It →
Lysosomal enzymes are predominantly .
Lysosomal enzymes are predominantly .
Tap to reveal answer
The lysosome is the "stomach" of the cell. It contains many hydrolytic enzymes to digest and recycle the monomers used to form old polymers. Remember the opposite of dehydration/condensation synthesis is hydrolysis. Hydrolysis reactions use water to break bonds in polymers, yielding monomers that can be recycled and reused in anabolic pathways.
The lysosome is the "stomach" of the cell. It contains many hydrolytic enzymes to digest and recycle the monomers used to form old polymers. Remember the opposite of dehydration/condensation synthesis is hydrolysis. Hydrolysis reactions use water to break bonds in polymers, yielding monomers that can be recycled and reused in anabolic pathways.
← Didn't Know|Knew It →
Which of the following best describes how a lysozyme works?
Which of the following best describes how a lysozyme works?
Tap to reveal answer
Lysozymes speed up by many times the hydrolysis of polysaccharides, by adding the water molecule to sugars linked in its enzyme-substrate complex. If left alone without the lysozyme, this hydrolysis would occur relatively infrequently, because it requires a large activation energy which would be supplied only by rare random collisions. The amino acid cleavage enzyme which uses the ping-pong mechanism is chymotrypsin. The enzyme which breaks nucleic acid phosophodiester bonds is phosphodiesterase. Fats are hydrolyzed by lipases, not lysozymes.
Lysozymes speed up by many times the hydrolysis of polysaccharides, by adding the water molecule to sugars linked in its enzyme-substrate complex. If left alone without the lysozyme, this hydrolysis would occur relatively infrequently, because it requires a large activation energy which would be supplied only by rare random collisions. The amino acid cleavage enzyme which uses the ping-pong mechanism is chymotrypsin. The enzyme which breaks nucleic acid phosophodiester bonds is phosphodiesterase. Fats are hydrolyzed by lipases, not lysozymes.
← Didn't Know|Knew It →
Which of the following lists the cytoskeletal filaments in order of increasing diameter?
Which of the following lists the cytoskeletal filaments in order of increasing diameter?
Tap to reveal answer
Actin filaments, also known as microfilaments, are flexible and bundle up, and are 5-9nm in diameter. Intermediate filaments, which can strengthen cells, are about 10nm. Microtubules, rigid and attached on one end to a centrosome, are 25nm.
Actin filaments, also known as microfilaments, are flexible and bundle up, and are 5-9nm in diameter. Intermediate filaments, which can strengthen cells, are about 10nm. Microtubules, rigid and attached on one end to a centrosome, are 25nm.
← Didn't Know|Knew It →
Which of the following correctly mentions the function of a common eukaryotic ligase?
Which of the following correctly mentions the function of a common eukaryotic ligase?
Tap to reveal answer
Mammalian DNA ligase I has this function, and there are other DNA ligases which perform it in other animals and eukaryotes (prokaryotes also have their own DNA ligases). All the other functions mentioned are done by other classes of enzymes, not ligases (i.e. hydrolases, aminotransferases, oxidoreductases, etc.).
Mammalian DNA ligase I has this function, and there are other DNA ligases which perform it in other animals and eukaryotes (prokaryotes also have their own DNA ligases). All the other functions mentioned are done by other classes of enzymes, not ligases (i.e. hydrolases, aminotransferases, oxidoreductases, etc.).
← Didn't Know|Knew It →
Which of the following lists the cytoskeletal filaments in order of increasing diameter?
Which of the following lists the cytoskeletal filaments in order of increasing diameter?
Tap to reveal answer
Actin filaments, also known as microfilaments, are flexible and bundle up, and are 5-9nm in diameter. Intermediate filaments, which can strengthen cells, are about 10nm. Microtubules, rigid and attached on one end to a centrosome, are 25nm.
Actin filaments, also known as microfilaments, are flexible and bundle up, and are 5-9nm in diameter. Intermediate filaments, which can strengthen cells, are about 10nm. Microtubules, rigid and attached on one end to a centrosome, are 25nm.
← Didn't Know|Knew It →
Enzymes can be regulated in a multitude of ways. One such way is by covalent modification, in which functional groups are attached to or removed from the enzyme. One such functional group that can be added to an enzyme is a phosphate group. Depending on the enzyme, addition of a phosphate group may increase or decrease that enzyme's activity. Which of the following is the general name of an enzyme that functions to add phosphate groups to its substrate?
Enzymes can be regulated in a multitude of ways. One such way is by covalent modification, in which functional groups are attached to or removed from the enzyme. One such functional group that can be added to an enzyme is a phosphate group. Depending on the enzyme, addition of a phosphate group may increase or decrease that enzyme's activity. Which of the following is the general name of an enzyme that functions to add phosphate groups to its substrate?
Tap to reveal answer
The correct answer is a kinase. Kinases are enzymes that couple the hydrolysis of ATP to the addition of a phosphate group to its substrate.
Phosphatase enzymes basically function oppositely to how kinases work. Phosphatases use water to hydrolyze phosphate groups off of their substrate.
Isomerase enzymes function to interconvert the structure of molecules from one isomer to another. This means that the substrate will remain with the same molecular formula, but it will have a difference in the connectivity of its bonds.
Ligases are enzymes that work by joining two molecules together.
Oxidoreductases are enzymes that act by catalyzing oxidation and reduction reactions, which involve the transfer of electrons from one molecule to another.
The correct answer is a kinase. Kinases are enzymes that couple the hydrolysis of ATP to the addition of a phosphate group to its substrate.
Phosphatase enzymes basically function oppositely to how kinases work. Phosphatases use water to hydrolyze phosphate groups off of their substrate.
Isomerase enzymes function to interconvert the structure of molecules from one isomer to another. This means that the substrate will remain with the same molecular formula, but it will have a difference in the connectivity of its bonds.
Ligases are enzymes that work by joining two molecules together.
Oxidoreductases are enzymes that act by catalyzing oxidation and reduction reactions, which involve the transfer of electrons from one molecule to another.
← Didn't Know|Knew It →
In the last step of glycolysis, what is the name of the enzyme that converts phosphoenolpyruvate into pyruvate?
In the last step of glycolysis, what is the name of the enzyme that converts phosphoenolpyruvate into pyruvate?
Tap to reveal answer
The last step of glycolysis, which involves the conversion of phosphoenolpyruvate into pyruvate, is catalyzed by the enzyme pyruvate kinase, yielding one pyruvate molecule and 1 ATP. The other kinases are involved in different steps of glycolysis.
The last step of glycolysis, which involves the conversion of phosphoenolpyruvate into pyruvate, is catalyzed by the enzyme pyruvate kinase, yielding one pyruvate molecule and 1 ATP. The other kinases are involved in different steps of glycolysis.
← Didn't Know|Knew It →
Which of the following enzymes catalyzes a reaction that is the functional opposite of the reaction catalyzed by kinases?
Which of the following enzymes catalyzes a reaction that is the functional opposite of the reaction catalyzed by kinases?
Tap to reveal answer
Kinases catalyze the attachment of phosphate groups to their substrates. Phosphatases specifically remove phosphate groups from their substrates, which is the opposite of the function of kinases. The other enzymes listed do not have functions that involve removal of phosphate groups.
Kinases catalyze the attachment of phosphate groups to their substrates. Phosphatases specifically remove phosphate groups from their substrates, which is the opposite of the function of kinases. The other enzymes listed do not have functions that involve removal of phosphate groups.
← Didn't Know|Knew It →
Which of the following is false regarding protein kinase A (PKA)?
Which of the following is false regarding protein kinase A (PKA)?
Tap to reveal answer
From it's name, we can assume that this kinase will add phosphate groups to its targets. The source of these phosphate groups is ATP. PKA has 2 catalytic, and 2 regulatory subunits. When PKA is activated, there is a conformational change that causes the regulatory subunits to fall off, and frees the catalytic (kinase) portions. When PKA is inhibited, the regulatory subunits bind to the catalytic subunits and prevent phosphorylation. PKA has many activators, but cAMP is one of the most robust and well studied activator. Therefore, cAMP binds to the regulatory subunits, but does not inhibit protein function.
From it's name, we can assume that this kinase will add phosphate groups to its targets. The source of these phosphate groups is ATP. PKA has 2 catalytic, and 2 regulatory subunits. When PKA is activated, there is a conformational change that causes the regulatory subunits to fall off, and frees the catalytic (kinase) portions. When PKA is inhibited, the regulatory subunits bind to the catalytic subunits and prevent phosphorylation. PKA has many activators, but cAMP is one of the most robust and well studied activator. Therefore, cAMP binds to the regulatory subunits, but does not inhibit protein function.
← Didn't Know|Knew It →
Kinases catalyze the phosphorylation of other proteins/substrates, which may trigger their activation. Phosphorylation involves the addition of a phosphate group to the target molecule. Amino acids with a(n) R-group are typically the substrates for phosphorylation.
Kinases catalyze the phosphorylation of other proteins/substrates, which may trigger their activation. Phosphorylation involves the addition of a phosphate group to the target molecule. Amino acids with a(n) R-group are typically the substrates for phosphorylation.
Tap to reveal answer
Polar R-groups, namely free hydroxyl groups on serine, threonine, and tyrosine, are the usual targets for phosphorylation because they are nucleophilic and can react with the phosphate group. Other R-groups are not as reactive and therefore are not ideal sites for phosphorylation.
Polar R-groups, namely free hydroxyl groups on serine, threonine, and tyrosine, are the usual targets for phosphorylation because they are nucleophilic and can react with the phosphate group. Other R-groups are not as reactive and therefore are not ideal sites for phosphorylation.
← Didn't Know|Knew It →