Protein Structure and Functions - Biochemistry
Card 1 of 392
Phosphoglucomutase is an enzyme seen in glycogen breakdown. It is responsible for converting glucose-1-phosphate ( ) to glucose-6-phosphate (
) to glucose-6-phosphate (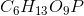 ).
).
Based on this action, to which enzyme class does phosphoglucomutase belong?
Phosphoglucomutase is an enzyme seen in glycogen breakdown. It is responsible for converting glucose-1-phosphate (
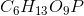
Based on this action, to which enzyme class does phosphoglucomutase belong?
Tap to reveal answer
Phosphoglucomutase is responsible for altering the position of the phosphate on the glucose from the "1" position to the "6" position. However, notice how the molecular formula for the product and the substrate are the same. Enzymes that rearrange the structure of a molecule in this manner are referred to as isomerase enzymes.
Phosphoglucomutase is responsible for altering the position of the phosphate on the glucose from the "1" position to the "6" position. However, notice how the molecular formula for the product and the substrate are the same. Enzymes that rearrange the structure of a molecule in this manner are referred to as isomerase enzymes.
← Didn't Know|Knew It →
Which proteins are generally water-soluble?
Which proteins are generally water-soluble?
Tap to reveal answer
In a globular protein, the amino acid chain can twist in a way that polar groups lie at the protein's surface. This allows the protein to interact with water and enhances the protein's solubility in water. This does not occur in fibrous proteins, so fibrous proteins are insoluble in water.
In a globular protein, the amino acid chain can twist in a way that polar groups lie at the protein's surface. This allows the protein to interact with water and enhances the protein's solubility in water. This does not occur in fibrous proteins, so fibrous proteins are insoluble in water.
← Didn't Know|Knew It →
Which of the following is false about actin filaments?
Which of the following is false about actin filaments?
Tap to reveal answer
Actin chain growth occurs at the (+) end of the chain, and nucleotide hydrolysis promotes dissociation of actin chains. 2 microfilaments of G-actin monomers make 1 filament of F-actin. However, actin filament assembly is powered by ATP, not GTP.
Actin chain growth occurs at the (+) end of the chain, and nucleotide hydrolysis promotes dissociation of actin chains. 2 microfilaments of G-actin monomers make 1 filament of F-actin. However, actin filament assembly is powered by ATP, not GTP.
← Didn't Know|Knew It →
An O-linked glycoprotein has a sugar attached to an oxygen atom on what amino acid(s)?
An O-linked glycoprotein has a sugar attached to an oxygen atom on what amino acid(s)?
Tap to reveal answer
An O-linked glycoprotein is a protein that has a sugar attached to it. It is called O-linked because the sugar is attached to an oxygen atom on either a threonine residue or a serine residue within the protein.
An O-linked glycoprotein is a protein that has a sugar attached to it. It is called O-linked because the sugar is attached to an oxygen atom on either a threonine residue or a serine residue within the protein.
← Didn't Know|Knew It →
If a protein is bonded to ubiquitin, this tells the cell that the protein should be .
If a protein is bonded to ubiquitin, this tells the cell that the protein should be .
Tap to reveal answer
When a protein is damaged, it can be tagged with the molecule, ubiquitin. This signals to the cell that the protein is no longer functioning properly and needs to be degraded.
When a protein is damaged, it can be tagged with the molecule, ubiquitin. This signals to the cell that the protein is no longer functioning properly and needs to be degraded.
← Didn't Know|Knew It →
HMGCoA reductase (3-hydroxy-3-methyl-glutaryl-CoA reductase) is the rate-limiting enzyme in cholesterol synthesis. Which of the following are true about the ubiquitination of this enzyme?
I. When cholesterol levels in the cell are high, the reductase binds to insulin-induced gene 1 proteins.
II. Binding to insulin induced gene 1 proteins leads to ubiquitination and proteasomal degradation of reductase.
III. Ubiquitination occurs through the binding of the C-terminal glycine of ubiquitin to the amino group of a lysine on the reductase.
IV. The enzyme tagged with ubiquitin is recognized by the proteasome where proteolysis occurs.
HMGCoA reductase (3-hydroxy-3-methyl-glutaryl-CoA reductase) is the rate-limiting enzyme in cholesterol synthesis. Which of the following are true about the ubiquitination of this enzyme?
I. When cholesterol levels in the cell are high, the reductase binds to insulin-induced gene 1 proteins.
II. Binding to insulin induced gene 1 proteins leads to ubiquitination and proteasomal degradation of reductase.
III. Ubiquitination occurs through the binding of the C-terminal glycine of ubiquitin to the amino group of a lysine on the reductase.
IV. The enzyme tagged with ubiquitin is recognized by the proteasome where proteolysis occurs.
Tap to reveal answer
HMGCoA reductase is the rate-limiting enzyme in cholesterol synthesis. The reductase is present on the endoplasmic reticulum membrane. When levels of its product, cholesterol, are high, the enzyme gets ubiquitinated and degraded in smaller peptides and amino acids. It first binds to insulin-induced gene 1 protein before ubiquitination.
HMGCoA reductase is the rate-limiting enzyme in cholesterol synthesis. The reductase is present on the endoplasmic reticulum membrane. When levels of its product, cholesterol, are high, the enzyme gets ubiquitinated and degraded in smaller peptides and amino acids. It first binds to insulin-induced gene 1 protein before ubiquitination.
← Didn't Know|Knew It →
Phosphoglucomutase is an enzyme seen in glycogen breakdown. It is responsible for converting glucose-1-phosphate ( ) to glucose-6-phosphate (
) to glucose-6-phosphate (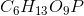 ).
).
Based on this action, to which enzyme class does phosphoglucomutase belong?
Phosphoglucomutase is an enzyme seen in glycogen breakdown. It is responsible for converting glucose-1-phosphate (
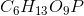
Based on this action, to which enzyme class does phosphoglucomutase belong?
Tap to reveal answer
Phosphoglucomutase is responsible for altering the position of the phosphate on the glucose from the "1" position to the "6" position. However, notice how the molecular formula for the product and the substrate are the same. Enzymes that rearrange the structure of a molecule in this manner are referred to as isomerase enzymes.
Phosphoglucomutase is responsible for altering the position of the phosphate on the glucose from the "1" position to the "6" position. However, notice how the molecular formula for the product and the substrate are the same. Enzymes that rearrange the structure of a molecule in this manner are referred to as isomerase enzymes.
← Didn't Know|Knew It →
Which of the following correctly mentions the function of a common eukaryotic ligase?
Which of the following correctly mentions the function of a common eukaryotic ligase?
Tap to reveal answer
Mammalian DNA ligase I has this function, and there are other DNA ligases which perform it in other animals and eukaryotes (prokaryotes also have their own DNA ligases). All the other functions mentioned are done by other classes of enzymes, not ligases (i.e. hydrolases, aminotransferases, oxidoreductases, etc.).
Mammalian DNA ligase I has this function, and there are other DNA ligases which perform it in other animals and eukaryotes (prokaryotes also have their own DNA ligases). All the other functions mentioned are done by other classes of enzymes, not ligases (i.e. hydrolases, aminotransferases, oxidoreductases, etc.).
← Didn't Know|Knew It →
How does an increase in carbon dioxide affect oxygen transport in hemoglobin?
How does an increase in carbon dioxide affect oxygen transport in hemoglobin?
Tap to reveal answer
A high carbon dioxide concentration will decrease the pH and produce the Bohr effect. These conditions will cause a slight conformational change in hemoglobin that results in a lower oxygen binding affinity. However, since the partial pressure of oxygen in the lungs in so high, most of the available oxygen will be loaded on to the hemoglobin anyway. Since the oxygen affinity is lowered, the hemoglobin will release the oxygen more freely, resulting in a greater oxygen to load in tissue. Functioning hemoglobin always has four cooperative subunits.
A high carbon dioxide concentration will decrease the pH and produce the Bohr effect. These conditions will cause a slight conformational change in hemoglobin that results in a lower oxygen binding affinity. However, since the partial pressure of oxygen in the lungs in so high, most of the available oxygen will be loaded on to the hemoglobin anyway. Since the oxygen affinity is lowered, the hemoglobin will release the oxygen more freely, resulting in a greater oxygen to load in tissue. Functioning hemoglobin always has four cooperative subunits.
← Didn't Know|Knew It →
Which of the following steps list in chronological order, the creation of a transport vesicle from a membrane via clathrin coating?
Which of the following steps list in chronological order, the creation of a transport vesicle from a membrane via clathrin coating?
Tap to reveal answer
The process of creating transport vesicles via clathrin coats proceeds in distinct steps. Before any budding occurs of the membrane, clathrin attaches to it, bound to adaptin which is attached to a transmembranal cargo receptor. (Cargo molecules are what trigger the creation of the vesicle in the first place.) The clathrin coating is believed to cause the membrane to bud. After the vesicle forms, dynamin uses GTP to pinch the vesicle off, and it is only then that the clathrin coat disassembles and we have a transport vesicle.
The process of creating transport vesicles via clathrin coats proceeds in distinct steps. Before any budding occurs of the membrane, clathrin attaches to it, bound to adaptin which is attached to a transmembranal cargo receptor. (Cargo molecules are what trigger the creation of the vesicle in the first place.) The clathrin coating is believed to cause the membrane to bud. After the vesicle forms, dynamin uses GTP to pinch the vesicle off, and it is only then that the clathrin coat disassembles and we have a transport vesicle.
← Didn't Know|Knew It →
Which of the following lists these molecules in order of increasing permeability to a lipid bilayer?
Which of the following lists these molecules in order of increasing permeability to a lipid bilayer?
Tap to reveal answer
Charged molecules do not permeate the lipid bilayer easily at all. So despite its small size, among our choices, a sodium ion passes least easily through. Polar molecules also have a hard (but less difficult) time passing through, and the larger the molecule, the harder that becomes, so after the sodium ion comes glucose, followed by water, which is polar but much smaller. Small, hydrophobic molecules -- such as carbon dioxide -- diffuse through most easily, because they can pass through the longest (hydrophobic) part of the membrane.
Charged molecules do not permeate the lipid bilayer easily at all. So despite its small size, among our choices, a sodium ion passes least easily through. Polar molecules also have a hard (but less difficult) time passing through, and the larger the molecule, the harder that becomes, so after the sodium ion comes glucose, followed by water, which is polar but much smaller. Small, hydrophobic molecules -- such as carbon dioxide -- diffuse through most easily, because they can pass through the longest (hydrophobic) part of the membrane.
← Didn't Know|Knew It →
Which type of transport does not require a protein?
Which type of transport does not require a protein?
Tap to reveal answer
There are two types of transport: passive transport and active transport. Active transport requires an expenditure of energy and a protein pump. Passive transport includes simple diffusion and facilitated diffusion. Diffusion is the movement of a substance from an area of high concentration to one of low concentration. Simple diffusion is the name for the diffusion process which does not require a protein; facilitated diffusion is the name for the diffusion process which requires a carrier protein for transport. Osmosis is the simple diffusion of water and thus does not require protein.
There are two types of transport: passive transport and active transport. Active transport requires an expenditure of energy and a protein pump. Passive transport includes simple diffusion and facilitated diffusion. Diffusion is the movement of a substance from an area of high concentration to one of low concentration. Simple diffusion is the name for the diffusion process which does not require a protein; facilitated diffusion is the name for the diffusion process which requires a carrier protein for transport. Osmosis is the simple diffusion of water and thus does not require protein.
← Didn't Know|Knew It →
Which of the following amino acids coordinates the heme ring in hemoglobin?
Which of the following amino acids coordinates the heme ring in hemoglobin?
Tap to reveal answer
In hemoglobin, the nitrogen group on the amino acid Histidine coordinates the heme ring in hemoglobin by binding to an iron atom located in the middle of the heme ring.
In hemoglobin, the nitrogen group on the amino acid Histidine coordinates the heme ring in hemoglobin by binding to an iron atom located in the middle of the heme ring.
← Didn't Know|Knew It →
A nonspecific hexagonal structure that functions to allow movement of molecules between adjacent cells is termed a(n) .
A nonspecific hexagonal structure that functions to allow movement of molecules between adjacent cells is termed a(n) .
Tap to reveal answer
A gap junction is a hexagonal protein that openly connects two adjacent cells. It is nonspecific, meaning it allows various different molecules and ions to travel through it. This type of transport is important for rapid communication between adjacent cells. For instance, gap junctions serve an important function in the heart - allowing the many cells present there to act as a functional syncytium. Integrins and tight junctions do not allow the passage of molecules between adjacent cells.
A gap junction is a hexagonal protein that openly connects two adjacent cells. It is nonspecific, meaning it allows various different molecules and ions to travel through it. This type of transport is important for rapid communication between adjacent cells. For instance, gap junctions serve an important function in the heart - allowing the many cells present there to act as a functional syncytium. Integrins and tight junctions do not allow the passage of molecules between adjacent cells.
← Didn't Know|Knew It →
GLUT (glucose transporters) proteins transport glucose from the blood to cells. Which of the following statements about them are true?
I. GLUT proteins are integral membrane proteins.
II . GLUT proteins have their amino and carboxyl termini on the extracellular side of the plasma membrane.
III. Binding of glucose to the transporter protein leads to a conformation change and the transport of glucose to the inner side of the membrane.
IV. GLUT-1, GLUT-3, and GLUT-4 proteins are ubiquitous; GLUT-2 is found in the liver, pancreas and kidney.
GLUT (glucose transporters) proteins transport glucose from the blood to cells. Which of the following statements about them are true?
I. GLUT proteins are integral membrane proteins.
II . GLUT proteins have their amino and carboxyl termini on the extracellular side of the plasma membrane.
III. Binding of glucose to the transporter protein leads to a conformation change and the transport of glucose to the inner side of the membrane.
IV. GLUT-1, GLUT-3, and GLUT-4 proteins are ubiquitous; GLUT-2 is found in the liver, pancreas and kidney.
Tap to reveal answer
GLUT are integral membrane proteins.The proteins cross the membrane having the amino and carboxyl termini on the cytoplasmic side of the plasma membrane. Binding of glucose to the transporter leads to a conformational change, transport of glucose to the other side of the membrane. GLUT-1, GLUT -3 and GLUT-4 are present in most tissues; GLUT-2 is found in the liver, pancreas and kidney.
GLUT are integral membrane proteins.The proteins cross the membrane having the amino and carboxyl termini on the cytoplasmic side of the plasma membrane. Binding of glucose to the transporter leads to a conformational change, transport of glucose to the other side of the membrane. GLUT-1, GLUT -3 and GLUT-4 are present in most tissues; GLUT-2 is found in the liver, pancreas and kidney.
← Didn't Know|Knew It →
A polypeptide when treated with trypsin yielded the following fragments:
(AM) (SAK) (YMPLWGIR)
The same polypeptide treated with chymotrypsin yielded the following fragments:
(MPLW) (GIRAM) (SAKY)
Which of the following displays the original sequence of the polypeptide?
A polypeptide when treated with trypsin yielded the following fragments:
(AM) (SAK) (YMPLWGIR)
The same polypeptide treated with chymotrypsin yielded the following fragments:
(MPLW) (GIRAM) (SAKY)
Which of the following displays the original sequence of the polypeptide?
Tap to reveal answer
This problem requires knowledge of the endopeptidase properties. Trypsin cleaves after the amino acids lysine and arginine, and chymotrypsin cleaves after the amino acids phenylalanine, tyrosine, and tryptophan.
Using this information, the slashes below indicate where each enzyme cleaved the polypeptide.
(AM) (SAK)/ (YMPLWGIR)/ trypsin
(MPLW)/ (GIRAM) (SAKY)/ chymotrypsin
Because the (AM) and (GIRAM) fragments have no slash after M in both the first and second treatment, one can conclude that this piece is at the end of the polypeptide. The other two pieces are sequenced by realizing that S starts the (SAK) and (SAKY) fragments and is never found in the middle of a fragment.
This problem requires knowledge of the endopeptidase properties. Trypsin cleaves after the amino acids lysine and arginine, and chymotrypsin cleaves after the amino acids phenylalanine, tyrosine, and tryptophan.
Using this information, the slashes below indicate where each enzyme cleaved the polypeptide.
(AM) (SAK)/ (YMPLWGIR)/ trypsin
(MPLW)/ (GIRAM) (SAKY)/ chymotrypsin
Because the (AM) and (GIRAM) fragments have no slash after M in both the first and second treatment, one can conclude that this piece is at the end of the polypeptide. The other two pieces are sequenced by realizing that S starts the (SAK) and (SAKY) fragments and is never found in the middle of a fragment.
← Didn't Know|Knew It →
Which of the following describes the unfolded protein response?
Which of the following describes the unfolded protein response?
Tap to reveal answer
There are 4 main steps in the unfolded protein response: (1) Translation of proteins is stopped, (2) Chaperones are recruited to the location of misfolded proteins, (3) Misfolded proteins are "tagged" with ubiquitin chains for degradation by the 26S proteasome, (4) if the above steps fail, the cell undergoes programmed cell death. Thus, all of the answers choices are affiliated with the unfolded protein response.
There are 4 main steps in the unfolded protein response: (1) Translation of proteins is stopped, (2) Chaperones are recruited to the location of misfolded proteins, (3) Misfolded proteins are "tagged" with ubiquitin chains for degradation by the 26S proteasome, (4) if the above steps fail, the cell undergoes programmed cell death. Thus, all of the answers choices are affiliated with the unfolded protein response.
← Didn't Know|Knew It →
Amino terminal - Ala - Lys - Glu - Phe - Phe - Ala - Leu - carboxyl terminal.
If the above primary sequence is cleaved by trypsin, on which amino acid will the new amino terminal be?
Amino terminal - Ala - Lys - Glu - Phe - Phe - Ala - Leu - carboxyl terminal.
If the above primary sequence is cleaved by trypsin, on which amino acid will the new amino terminal be?
Tap to reveal answer
Trypsin will cleave the primary sequence after the lysine residue (on its carboxyl side). Thus, Lys will be the new carboxyl terminal and Glu will be the new amino terminal. Remember that a protein's primary sequence is written from N to C.
Trypsin will cleave the primary sequence after the lysine residue (on its carboxyl side). Thus, Lys will be the new carboxyl terminal and Glu will be the new amino terminal. Remember that a protein's primary sequence is written from N to C.
← Didn't Know|Knew It →
Which of the following proteases would cleave lysine at the carbonyl side?
Which of the following proteases would cleave lysine at the carbonyl side?
Tap to reveal answer
Trypsin will cleave lysine and arginine at the carbonyl side. Chymotrypsin will cleave phenylalanine, tyrosine, and tryptophan at the carbonyl side. Pepsin will cleave leucine, phenylalanine, tryptophan, and tyrosine at the amino side. Pepsinogen is the inactive form of pepsin, which gets activated via cleavage by hydrochloric acid in the stomach.
Trypsin will cleave lysine and arginine at the carbonyl side. Chymotrypsin will cleave phenylalanine, tyrosine, and tryptophan at the carbonyl side. Pepsin will cleave leucine, phenylalanine, tryptophan, and tyrosine at the amino side. Pepsinogen is the inactive form of pepsin, which gets activated via cleavage by hydrochloric acid in the stomach.
← Didn't Know|Knew It →
What is the result of chymotrypsin being added to the peptide shown?
Gly-Ala-Pro-Tyr-His-Cys-Gly-Phe-Gly-Gly-Asn
What is the result of chymotrypsin being added to the peptide shown?
Gly-Ala-Pro-Tyr-His-Cys-Gly-Phe-Gly-Gly-Asn
Tap to reveal answer
Chymotryspin cleaves Phe, Trp, and Tyr at the carbonyl side. This means that there will be a cleavage after any of these three amino acids appears. This results in Gly-Ala-Pro-Tyr, His-Cys-Gly-Phe, Gly-Gly-Asn.
Chymotryspin cleaves Phe, Trp, and Tyr at the carbonyl side. This means that there will be a cleavage after any of these three amino acids appears. This results in Gly-Ala-Pro-Tyr, His-Cys-Gly-Phe, Gly-Gly-Asn.
← Didn't Know|Knew It →