0%
0 / 12 answered
Solubility and Equilibrium Practice Test
•12 QuestionsQuestion
1 / 12
Q1
Four different insoluble salts are added to an aqueous solution in equal amounts. Assume that the salts do not interact with one another in any way. The following list shows the salts and their solubility product constants.






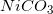

Which of the following ions will have the highest concentration in the solution?
Four different insoluble salts are added to an aqueous solution in equal amounts. Assume that the salts do not interact with one another in any way. The following list shows the salts and their solubility product constants.



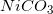
Which of the following ions will have the highest concentration in the solution?
 .
.