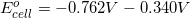Thermodynamics
Help Questions
AP Chemistry › Thermodynamics
A chemistry student is trying to calculate how long it will take a power source of 






There is not enough information to determine the amount of time needed for the process described
Explanation
In order to solve this problem, we'll need to break it up into steps.
Step 1: Calculate the amount of energy necessary to raise the sample of ice from 

Step 2: Calculate the amount of energy necessary to convert the sample at 
Step 3: Calculate the amount of energy necessary to convert the sample of water from 

Step 4: Sum the amount of energy from the previous 3 steps. This value is the total amount of energy for the entire process.
Step 5: Now that we know the total amount of energy needed for the process, we need to calculate the time based on the amount of power provided.
Which of the following is a law of thermodynamics?
ΔH (system) + ΔH (surroundings) = ΔH (universe)
ΔS (system) + ΔS (surroundings) = ΔS (universe)
ΔE (surroundings) = ΔE (system)
The entropy of the universe is always decreasing
Explanation
The second law of thermodynamics states that the entropy of the system and the entropy of the surroundings is equal to the entropy of the universe. The rest of the answer choices are not one of the fundamental laws of themodynamics.
1. 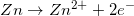
2. 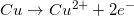
3.
What is 




Explanation
There are two concepts to consider in this problem. First, the question asks for the 


For the following cell reaction:
2 Al (s) + 3 Mn2+ (aq) -> 2 Al3+ (aq) + 3 Mn (s)
predict if the cell potential Ecell will be larger, or smaller than Eocell for the following conditions: \[Al3+\] = 1.0 M; \[Mn2+\] = 5.0 M.
Larger
Smaller
Identical
Can not be determined
Explanation
At these concentrations Q will become smaller, and thus log Q will become smaller. This will give rise to a larger cell potential.
Calculate the standard cell potential of the following reaction:
Cd(s) + MnO2 (s) + 4 H+ (aq) + -> Cd2+ (aq) + Mn2+ (aq) + 2 H2O (l)
Given:
MnO2 (s) + 4 H+ (aq) + 2e- -> Mn2+ (aq) + 2 H2O (l) Eo = 1.23 V
Cd2+ (aq) + 2 e- -> Cd (s) Eo = -0.40 V
1.63 V
0.83 V
-1.63 V
-0.83 V
0.0 V
Explanation
Eocell = Eo cathode - Eoanode
Eocell = 1.23 – (-0.40) = 1.63 V
The following galvanic cell is created:

Which of the following takes place at the cathode?
Gold ions receive electrons
Copper loses electrons
Gold loses electrons
Copper gains electrons
Explanation
Reduction always takes place at the cathode. This means that a substance is receiving electrons.
In the reaction, the gold ions are receiving electrons in order to create gold atoms. This takes place at the cathode.
Copper ions and gold are products, and will not be oxidized or reduced. Copper is reduced at the anode during this reaction.
How many grams of Cr can be obtained by the electrolysis of a Cr(NO3)3 if 10 amps are passed through the cell for 6 hours?
38.8 g
19.4 g
103 g
12.5 g
56.3 g
Explanation
The following galvanic cell is created:

Which of the following takes place at the cathode?
Gold ions receive electrons
Copper loses electrons
Gold loses electrons
Copper gains electrons
Explanation
Reduction always takes place at the cathode. This means that a substance is receiving electrons.
In the reaction, the gold ions are receiving electrons in order to create gold atoms. This takes place at the cathode.
Copper ions and gold are products, and will not be oxidized or reduced. Copper is reduced at the anode during this reaction.
A chemistry student is trying to calculate how long it will take a power source of 






There is not enough information to determine the amount of time needed for the process described
Explanation
In order to solve this problem, we'll need to break it up into steps.
Step 1: Calculate the amount of energy necessary to raise the sample of ice from 

Step 2: Calculate the amount of energy necessary to convert the sample at 
Step 3: Calculate the amount of energy necessary to convert the sample of water from 

Step 4: Sum the amount of energy from the previous 3 steps. This value is the total amount of energy for the entire process.
Step 5: Now that we know the total amount of energy needed for the process, we need to calculate the time based on the amount of power provided.
How many grams of Cr can be obtained by the electrolysis of a Cr(NO3)3 if 10 amps are passed through the cell for 6 hours?
38.8 g
19.4 g
103 g
12.5 g
56.3 g