Thermodynamics - AP Chemistry
Card 1 of 1235
Consider an electrochemical cell that has the following overall reaction:
2 H+(aq) + Sn (s) -> Sn2+ (aq) + H2 (aq)
Which of the following changes would alter the measured cell potential?
Consider an electrochemical cell that has the following overall reaction:
2 H+(aq) + Sn (s) -> Sn2+ (aq) + H2 (aq)
Which of the following changes would alter the measured cell potential?
Tap to reveal answer

All of these changes would change Q and thus change the measured cell potential.
All of these changes would change Q and thus change the measured cell potential.
← Didn't Know|Knew It →
For the following cell reaction:
2 Al (s) + 3 Mn2+ (aq) -> 2 Al3+ (aq) + 3 Mn (s)
predict if the cell potential Ecell will be larger, or smaller than Eocell for the following conditions:
\[Al3+\] = 2.0 M; \[Mn2+\] = 1.0 M.
For the following cell reaction:
2 Al (s) + 3 Mn2+ (aq) -> 2 Al3+ (aq) + 3 Mn (s)
predict if the cell potential Ecell will be larger, or smaller than Eocell for the following conditions:
\[Al3+\] = 2.0 M; \[Mn2+\] = 1.0 M.
Tap to reveal answer

Altering these conditions would increase Q, and thus result in a decrease in the measured cell potential.
Altering these conditions would increase Q, and thus result in a decrease in the measured cell potential.
← Didn't Know|Knew It →
Determine the Ecell for the following reaction at 25 C:
Zn (s) + 2 VO2+ (aq) + 4 H+ -> 2 VO2+ (aq) + Zn2+(aq) + 2 H2O (l)
Given that:
VO2+ (aq) + 2 H+ (aq) + e- -> VO2+(aq) + H2O (l) Eo = 1.00 V
Zn2+ (aq) + 2 e--> Zn (s) Eo = -0.76 V
And
\[ VO2+\] = 2.0 M; \[H+\] = 0.50 M; \[VO2+\] = 1.0 x 10-2M; \[Zn2+\] = 1.0 x 10-1M
Determine the Ecell for the following reaction at 25 C:
Zn (s) + 2 VO2+ (aq) + 4 H+ -> 2 VO2+ (aq) + Zn2+(aq) + 2 H2O (l)
Given that:
VO2+ (aq) + 2 H+ (aq) + e- -> VO2+(aq) + H2O (l) Eo = 1.00 V
Zn2+ (aq) + 2 e--> Zn (s) Eo = -0.76 V
And
\[ VO2+\] = 2.0 M; \[H+\] = 0.50 M; \[VO2+\] = 1.0 x 10-2M; \[Zn2+\] = 1.0 x 10-1M
Tap to reveal answer
← Didn't Know|Knew It →
For the following cell reaction:
2 Al (s) + 3 Mn2+ (aq) -> 2 Al3+ (aq) + 3 Mn (s)
predict if the cell potential Ecell will be larger, or smaller than Eocell for the following conditions: \[Al3+\] = 1.0 M; \[Mn2+\] = 5.0 M.
For the following cell reaction:
2 Al (s) + 3 Mn2+ (aq) -> 2 Al3+ (aq) + 3 Mn (s)
predict if the cell potential Ecell will be larger, or smaller than Eocell for the following conditions: \[Al3+\] = 1.0 M; \[Mn2+\] = 5.0 M.
Tap to reveal answer

At these concentrations Q will become smaller, and thus log Q will become smaller. This will give rise to a larger cell potential.
At these concentrations Q will become smaller, and thus log Q will become smaller. This will give rise to a larger cell potential.
← Didn't Know|Knew It →
What is the cell potential of the following cell:
Zn (s) + 2 H+(aq) -> Zn2+ (aq) + H2 (g) Eo = 0.76 V
When the \[Zn2+\] = 1.0 M; PH2 = 1 atm, and the pH in the cathode is 5.2?
What is the cell potential of the following cell:
Zn (s) + 2 H+(aq) -> Zn2+ (aq) + H2 (g) Eo = 0.76 V
When the \[Zn2+\] = 1.0 M; PH2 = 1 atm, and the pH in the cathode is 5.2?
Tap to reveal answer
← Didn't Know|Knew It →
Calculate the standard cell potential of the following reaction:
Cd(s) + MnO2 (s) + 4 H+ (aq) + -> Cd2+ (aq) + Mn2+ (aq) + 2 H2O (l)
Given:
MnO2 (s) + 4 H+ (aq) + 2e- -> Mn2+ (aq) + 2 H2O (l) Eo = 1.23 V
Cd2+ (aq) + 2 e- -> Cd (s) Eo = -0.40 V
Calculate the standard cell potential of the following reaction:
Cd(s) + MnO2 (s) + 4 H+ (aq) + -> Cd2+ (aq) + Mn2+ (aq) + 2 H2O (l)
Given:
MnO2 (s) + 4 H+ (aq) + 2e- -> Mn2+ (aq) + 2 H2O (l) Eo = 1.23 V
Cd2+ (aq) + 2 e- -> Cd (s) Eo = -0.40 V
Tap to reveal answer
Eocell = Eo cathode - Eoanode
Eocell = 1.23 – (-0.40) = 1.63 V
Eocell = Eo cathode - Eoanode
Eocell = 1.23 – (-0.40) = 1.63 V
← Didn't Know|Knew It →
Calculate the standard cell potential of the following reaction:
Zn (s) + Cu2+ (aq) -> Zn2+ (aq) + Cu (s)
Given:
Zn2+(aq)+ 2 e--> Zn (s) Eo = -0.76 V
Cu2+(aq)+ 2 e--> Cu (s) Eo = 0.34 V
Calculate the standard cell potential of the following reaction:
Zn (s) + Cu2+ (aq) -> Zn2+ (aq) + Cu (s)
Given:
Zn2+(aq)+ 2 e--> Zn (s) Eo = -0.76 V
Cu2+(aq)+ 2 e--> Cu (s) Eo = 0.34 V
Tap to reveal answer
Eocell = Eo cathode - Eoanode
Eocell = 0.34 – (-0.76) = 1.10 V
Eocell = Eo cathode - Eoanode
Eocell = 0.34 – (-0.76) = 1.10 V
← Didn't Know|Knew It →
Calculate the standard cell potential of the following reaction:
Zn (s) + 2 Ag1+ (aq) -> Zn2+ (aq) + 2 Ag (s)
Given:
Zn2+(aq)+ 2 e--> Zn (s) Eo = -0.76 V
Ag1+(aq)+ 1 e--> Ag (s) Eo = 0.80 V
Calculate the standard cell potential of the following reaction:
Zn (s) + 2 Ag1+ (aq) -> Zn2+ (aq) + 2 Ag (s)
Given:
Zn2+(aq)+ 2 e--> Zn (s) Eo = -0.76 V
Ag1+(aq)+ 1 e--> Ag (s) Eo = 0.80 V
Tap to reveal answer
Eocell = Eo cathode - Eoanode
Eocell = 0.80 – (-0.76) = 1.56 V
Eocell = Eo cathode - Eoanode
Eocell = 0.80 – (-0.76) = 1.56 V
← Didn't Know|Knew It →
Calculate the standard cell potential of the following reaction:
3 F2 (g) + 2 Au (s) -> 6 F- (aq) + 2 Au3+
Given:
F2 (g) + 2 e- -> 2 F- (aq) Eo = 2.87 V
Au3+(aq)+ 3 e--> Au (s) Eo = 1.50 V
Calculate the standard cell potential of the following reaction:
3 F2 (g) + 2 Au (s) -> 6 F- (aq) + 2 Au3+
Given:
F2 (g) + 2 e- -> 2 F- (aq) Eo = 2.87 V
Au3+(aq)+ 3 e--> Au (s) Eo = 1.50 V
Tap to reveal answer
Eocell = Eo cathode - Eoanode
Eocell = 2.87 – (1.50) = 1.37 V
Eocell = Eo cathode - Eoanode
Eocell = 2.87 – (1.50) = 1.37 V
← Didn't Know|Knew It →
H2O ice melts to liquid H2O \[Heat is added\]
Which of the following is correct?
I. Δ H is positive
II. ΔS is positive
III. ΔH is negative
IV. ΔS is negative
H2O ice melts to liquid H2O \[Heat is added\]
Which of the following is correct?
I. Δ H is positive
II. ΔS is positive
III. ΔH is negative
IV. ΔS is negative
Tap to reveal answer
When heat is added, enthalpy increases, ΔH refers to enthalpy
ΔS refers to entropy, which is disorder; disorder increases as it moves from solid to liquid to gas state.
As ice goes to liquid water, entropy increases
When heat is added, enthalpy increases, ΔH refers to enthalpy
ΔS refers to entropy, which is disorder; disorder increases as it moves from solid to liquid to gas state.
As ice goes to liquid water, entropy increases
← Didn't Know|Knew It →
Calculate ΔH for the following reaction:
CH4 (g) + O2 (g) ⇌ CO2 (g) + H2O (l)
Compound ΔH
CH4 (g) -74.8 kJ/mol
H2O (l) -285.8 kJ/mol
CO2 (g) -393.5 kJ/mol
Calculate ΔH for the following reaction:
CH4 (g) + O2 (g) ⇌ CO2 (g) + H2O (l)
Compound ΔH
CH4 (g) -74.8 kJ/mol
H2O (l) -285.8 kJ/mol
CO2 (g) -393.5 kJ/mol
Tap to reveal answer
ΔH = Σ ΔHf products - Σ ΔHf reactants
ΔHf O2 or any element is 0
First step is to balance the equation:
CH4 (g) + O2 (g) ⇌ CO2 (g) + 2H2O (l)
ΔH = Σ ΔHf products - Σ ΔHf reactants
= \[-393.5 kJ/mol + 2(-285.8) kJ/mol\] - (-74.8 kJ/mol)
= -890.3 kJ/mol
ΔH = Σ ΔHf products - Σ ΔHf reactants
ΔHf O2 or any element is 0
First step is to balance the equation:
CH4 (g) + O2 (g) ⇌ CO2 (g) + 2H2O (l)
ΔH = Σ ΔHf products - Σ ΔHf reactants
= \[-393.5 kJ/mol + 2(-285.8) kJ/mol\] - (-74.8 kJ/mol)
= -890.3 kJ/mol
← Didn't Know|Knew It →
Which of the following is a true statement regarding the energy involved in the formation of Methane, CH4, from graphite C(s) and H2(g) ?
Which of the following is a true statement regarding the energy involved in the formation of Methane, CH4, from graphite C(s) and H2(g) ?
Tap to reveal answer
This problem requires a knowledge of the energy involved when bonds are broken versus when bonds are formed. Energy is released when bonds are formed, and energy is consumed when bonds are broken. Here, methane is being formed from C and H2. Since the carbon source, graphite, is monoatomic the carbon will be involved only in the formation of C–H bonds on it's way to forming methane. The H however exists as H2 so bonds will need to be broken to allow for C–H bonds to form. So first energy is consumed to break the H–H bonds, it is then released when the C–H bonds form.
This problem requires a knowledge of the energy involved when bonds are broken versus when bonds are formed. Energy is released when bonds are formed, and energy is consumed when bonds are broken. Here, methane is being formed from C and H2. Since the carbon source, graphite, is monoatomic the carbon will be involved only in the formation of C–H bonds on it's way to forming methane. The H however exists as H2 so bonds will need to be broken to allow for C–H bonds to form. So first energy is consumed to break the H–H bonds, it is then released when the C–H bonds form.
← Didn't Know|Knew It →
The formation of nitrogen dioxide is a two step process.




The net reaction is  .
.
What is the change in enthalpy when creating four moles of nitrogen dioxide?
The formation of nitrogen dioxide is a two step process.


The net reaction is 
What is the change in enthalpy when creating four moles of nitrogen dioxide?
Tap to reveal answer
Hess's law states that the change in enthalpy for a total reaction can be considered equal to the sum of the enthalpy changes for every step involved in the reaction. In other words, we can determine the enthalpy change for nitrogen dioxide by adding the enthalpy changes for both steps involved in its formation.


This gives us the total change in enthalpy for the listed reaction,  . Because the question asks for the enthalpy change for four moles of nitrogen dioxide, the value must be doubled. The reaction only produces two moles of nitrogen dioxide.
. Because the question asks for the enthalpy change for four moles of nitrogen dioxide, the value must be doubled. The reaction only produces two moles of nitrogen dioxide.


Hess's law states that the change in enthalpy for a total reaction can be considered equal to the sum of the enthalpy changes for every step involved in the reaction. In other words, we can determine the enthalpy change for nitrogen dioxide by adding the enthalpy changes for both steps involved in its formation.
This gives us the total change in enthalpy for the listed reaction, 
← Didn't Know|Knew It →
Which of the following statements is true concerning a chemical reaction?
Which of the following statements is true concerning a chemical reaction?
Tap to reveal answer
When a chemical reaction is represented graphically, we see that the enthalpy change is reversed between the forward and reverse reactions. If a reaction produces energy in a forward process, it will require an input of energy in the reverse process, and vice versa.


A catalyst only affects the rate of a chemical reaction; it does not affect the equilibrium. Finally, exothermic reactions are not always spontaneous, but will have lower activation of energies compared to endothermic reactions.
When a chemical reaction is represented graphically, we see that the enthalpy change is reversed between the forward and reverse reactions. If a reaction produces energy in a forward process, it will require an input of energy in the reverse process, and vice versa.
A catalyst only affects the rate of a chemical reaction; it does not affect the equilibrium. Finally, exothermic reactions are not always spontaneous, but will have lower activation of energies compared to endothermic reactions.
← Didn't Know|Knew It →






What is the change in enthalpy for the given reaction?




What is the change in enthalpy for the given reaction?
Tap to reveal answer
The change in enthalpy is calculated by:

When  cannot be measured, it can be calculated from known reactions. In this case the known reactions are given.
cannot be measured, it can be calculated from known reactions. In this case the known reactions are given.






Since the reactions are in the correct order, adding all the  values together can be used to calculate the
values together can be used to calculate the  of the reaction.
of the reaction.



The change in enthalpy is calculated by:
When 



Since the reactions are in the correct order, adding all the 

← Didn't Know|Knew It →






What is the enthalpy of the following reaction?




What is the enthalpy of the following reaction?
Tap to reveal answer
The change in enthalpy is calculated by:

When  cannot be measured, it can be calculated from known enthalpies of formation.
cannot be measured, it can be calculated from known enthalpies of formation.






It is important to first balance the reaction before performing calculations. The coefficients are important in determining the change in enthalpy of a reaction. For this particular reaction, since there are two moles of product, the enthalpy of formation for  must be multiplied by two.
must be multiplied by two.




The change in enthalpy is calculated by:
When 



It is important to first balance the reaction before performing calculations. The coefficients are important in determining the change in enthalpy of a reaction. For this particular reaction, since there are two moles of product, the enthalpy of formation for 
← Didn't Know|Knew It →






What is the change in enthalpy for the following reaction?




What is the change in enthalpy for the following reaction?
Tap to reveal answer
The change in enthalpy is calculated by:

When  cannot be measured, it can be calculated from known enthalpies of formation.
cannot be measured, it can be calculated from known enthalpies of formation.






It is important to first balance the reaction before performing calculations. The coefficients are important in determining the change in enthalpy of a reaction.




The change in enthalpy is calculated by:
When 



It is important to first balance the reaction before performing calculations. The coefficients are important in determining the change in enthalpy of a reaction.
← Didn't Know|Knew It →






What is the change in enthalpy for the following reaction?




What is the change in enthalpy for the following reaction?
Tap to reveal answer
The change in enthalpy is calculated by:

When  cannot be measured, it can be calculated from known enthalpies of formation.
cannot be measured, it can be calculated from known enthalpies of formation.






It is important to first balance the reaction before performing calculations. The coefficients are important in determining the change in enthalpy of a reaction.




The change in enthalpy is calculated by:
When 



It is important to first balance the reaction before performing calculations. The coefficients are important in determining the change in enthalpy of a reaction.
← Didn't Know|Knew It →
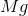



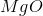

What is the change in enthalpy for the following reaction?

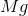

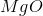
What is the change in enthalpy for the following reaction?
Tap to reveal answer
The change in enthalpy is calculated by:

When  cannot be measured, it can be calculated from known enthalpies of formation.
cannot be measured, it can be calculated from known enthalpies of formation.
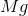



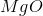

It is important to first balance the reaction before performing calculations. The coefficients are important in determining the change in enthalpy of a reaction.




The change in enthalpy is calculated by:
When 
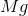

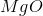
It is important to first balance the reaction before performing calculations. The coefficients are important in determining the change in enthalpy of a reaction.
← Didn't Know|Knew It →








What is the change in enthalpy for the following reaction?





What is the change in enthalpy for the following reaction?
Tap to reveal answer
The change in enthalpy is calculated by:

When  cannot be measured, it can be calculated from known enthalpies of formation.
cannot be measured, it can be calculated from known enthalpies of formation.








It is important to first balance the reaction before performing calculations. The coefficients are important in determining the change in enthalpy of a reaction.




The change in enthalpy is calculated by:
When 




It is important to first balance the reaction before performing calculations. The coefficients are important in determining the change in enthalpy of a reaction.
← Didn't Know|Knew It →





![= 1.76 V - $\frac{0.0592}{2}$: log : $\frac{[1.0 x $10^{-2}$$ $]^2$ [1.0 x $10^{-1}$ $]}{[2.0]^2$ $[0.5]^4$} = 1.89](http://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1172348/gif.latex)


![[H^+ ] = $10^{-pH}$= $10^{-5.2}$ = 6.31 x $10^{-6}$](http://vt-vtwa-assets.varsitytutors.com/vt-vtwa/uploads/formula_image/image/1172342/gif.latex)

