Reduction Potential - AP Chemistry
Card 1 of 42
Standard Reduction Potentials
Cr3+(aq) + 3e– → Cr (s) –0.74 V
Cu2+(aq) +2e– → Cu (s) 0.34 V
Consider the following reaction
Cu(s) + Cr3+(aq) ⇌ Cu2+(aq) + Cr(s)
What is the Eo Cell for the reaction?
Standard Reduction Potentials
Cr3+(aq) + 3e– → Cr (s) –0.74 V
Cu2+(aq) +2e– → Cu (s) 0.34 V
Consider the following reaction
Cu(s) + Cr3+(aq) ⇌ Cu2+(aq) + Cr(s)
What is the Eo Cell for the reaction?
Tap to reveal answer
You do not multiply the coefficents that you need to balance to the Eo cell; you just have to see that the copper is being oxidized, so the sign changes (0.34 → –0.34) and the Cr is being reduced so the sign doesn't change. Then, just add the Eo cells: –0.74 + (–0.34) = –1.08.
You do not multiply the coefficents that you need to balance to the Eo cell; you just have to see that the copper is being oxidized, so the sign changes (0.34 → –0.34) and the Cr is being reduced so the sign doesn't change. Then, just add the Eo cells: –0.74 + (–0.34) = –1.08.
← Didn't Know|Knew It →
The standard reduction potentials for certain metals are listed below:
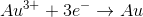
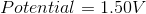

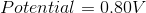

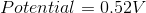




What is the standard potential of a cell in which copper is reduced by iron?
The standard reduction potentials for certain metals are listed below:
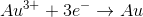




What is the standard potential of a cell in which copper is reduced by iron?
Tap to reveal answer
In order to find the potential of a galvanic cell in which copper is reduced by iron, we need to combine the two half reactions for the metals in question.
Since copper is reduced in the net ionic equation, we can use the reduction potential seen in the table.


Iron must be oxidized in order to reduce the copper. As a result, we must use the reverse reaction in the table. Remember that the table only gives reduction potentials; you will frequently need to charge the reaction to find an oxidation potential.


Notice how the potential for the half reaction has been switched as well. Combining these two reactions gives the following balanced reaction, once the electron transfer is balanced.

Reduction potentials are intensive properties, so we do not multiply the potential of the copper half reaction by two. The total potential is simply the addition of both half reaction potentials.

In order to find the potential of a galvanic cell in which copper is reduced by iron, we need to combine the two half reactions for the metals in question.
Since copper is reduced in the net ionic equation, we can use the reduction potential seen in the table.

Iron must be oxidized in order to reduce the copper. As a result, we must use the reverse reaction in the table. Remember that the table only gives reduction potentials; you will frequently need to charge the reaction to find an oxidation potential.

Notice how the potential for the half reaction has been switched as well. Combining these two reactions gives the following balanced reaction, once the electron transfer is balanced.
Reduction potentials are intensive properties, so we do not multiply the potential of the copper half reaction by two. The total potential is simply the addition of both half reaction potentials.
← Didn't Know|Knew It →
The standard reduction potentials for some metals are listed below:










Which of the following metals is the strongest reducing agent?
The standard reduction potentials for some metals are listed below:





Which of the following metals is the strongest reducing agent?
Tap to reveal answer
The reducing agent is the metal that will be oxidized in the reaction. Since all of the half reactions shown are reduction potentials, we need to switch the half reactions in order to determine which results in the greatest cell potential when the metal is oxidized. Iron will result in a cell potential of 0.44 volts when oxidized, which makes it the strongest reducing agent on the list.
The reducing agent is the metal that will be oxidized in the reaction. Since all of the half reactions shown are reduction potentials, we need to switch the half reactions in order to determine which results in the greatest cell potential when the metal is oxidized. Iron will result in a cell potential of 0.44 volts when oxidized, which makes it the strongest reducing agent on the list.
← Didn't Know|Knew It →
Standard Reduction Potentials
Cr3+(aq) + 3e– → Cr (s) –0.74 V
Cu2+(aq) +2e– → Cu (s) 0.34 V
Consider the following reaction
Cu(s) + Cr3+(aq) ⇌ Cu2+(aq) + Cr(s)
What is the Eo Cell for the reaction?
Standard Reduction Potentials
Cr3+(aq) + 3e– → Cr (s) –0.74 V
Cu2+(aq) +2e– → Cu (s) 0.34 V
Consider the following reaction
Cu(s) + Cr3+(aq) ⇌ Cu2+(aq) + Cr(s)
What is the Eo Cell for the reaction?
Tap to reveal answer
You do not multiply the coefficents that you need to balance to the Eo cell; you just have to see that the copper is being oxidized, so the sign changes (0.34 → –0.34) and the Cr is being reduced so the sign doesn't change. Then, just add the Eo cells: –0.74 + (–0.34) = –1.08.
You do not multiply the coefficents that you need to balance to the Eo cell; you just have to see that the copper is being oxidized, so the sign changes (0.34 → –0.34) and the Cr is being reduced so the sign doesn't change. Then, just add the Eo cells: –0.74 + (–0.34) = –1.08.
← Didn't Know|Knew It →
The standard reduction potentials for certain metals are listed below:
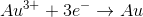
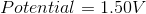

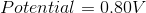

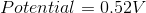




What is the standard potential of a cell in which copper is reduced by iron?
The standard reduction potentials for certain metals are listed below:
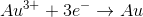




What is the standard potential of a cell in which copper is reduced by iron?
Tap to reveal answer
In order to find the potential of a galvanic cell in which copper is reduced by iron, we need to combine the two half reactions for the metals in question.
Since copper is reduced in the net ionic equation, we can use the reduction potential seen in the table.


Iron must be oxidized in order to reduce the copper. As a result, we must use the reverse reaction in the table. Remember that the table only gives reduction potentials; you will frequently need to charge the reaction to find an oxidation potential.


Notice how the potential for the half reaction has been switched as well. Combining these two reactions gives the following balanced reaction, once the electron transfer is balanced.

Reduction potentials are intensive properties, so we do not multiply the potential of the copper half reaction by two. The total potential is simply the addition of both half reaction potentials.

In order to find the potential of a galvanic cell in which copper is reduced by iron, we need to combine the two half reactions for the metals in question.
Since copper is reduced in the net ionic equation, we can use the reduction potential seen in the table.

Iron must be oxidized in order to reduce the copper. As a result, we must use the reverse reaction in the table. Remember that the table only gives reduction potentials; you will frequently need to charge the reaction to find an oxidation potential.

Notice how the potential for the half reaction has been switched as well. Combining these two reactions gives the following balanced reaction, once the electron transfer is balanced.
Reduction potentials are intensive properties, so we do not multiply the potential of the copper half reaction by two. The total potential is simply the addition of both half reaction potentials.
← Didn't Know|Knew It →
The standard reduction potentials for some metals are listed below:










Which of the following metals is the strongest reducing agent?
The standard reduction potentials for some metals are listed below:





Which of the following metals is the strongest reducing agent?
Tap to reveal answer
The reducing agent is the metal that will be oxidized in the reaction. Since all of the half reactions shown are reduction potentials, we need to switch the half reactions in order to determine which results in the greatest cell potential when the metal is oxidized. Iron will result in a cell potential of 0.44 volts when oxidized, which makes it the strongest reducing agent on the list.
The reducing agent is the metal that will be oxidized in the reaction. Since all of the half reactions shown are reduction potentials, we need to switch the half reactions in order to determine which results in the greatest cell potential when the metal is oxidized. Iron will result in a cell potential of 0.44 volts when oxidized, which makes it the strongest reducing agent on the list.
← Didn't Know|Knew It →
Standard Reduction Potentials
Cr3+(aq) + 3e– → Cr (s) –0.74 V
Cu2+(aq) +2e– → Cu (s) 0.34 V
Consider the following reaction
Cu(s) + Cr3+(aq) ⇌ Cu2+(aq) + Cr(s)
What is the Eo Cell for the reaction?
Standard Reduction Potentials
Cr3+(aq) + 3e– → Cr (s) –0.74 V
Cu2+(aq) +2e– → Cu (s) 0.34 V
Consider the following reaction
Cu(s) + Cr3+(aq) ⇌ Cu2+(aq) + Cr(s)
What is the Eo Cell for the reaction?
Tap to reveal answer
You do not multiply the coefficents that you need to balance to the Eo cell; you just have to see that the copper is being oxidized, so the sign changes (0.34 → –0.34) and the Cr is being reduced so the sign doesn't change. Then, just add the Eo cells: –0.74 + (–0.34) = –1.08.
You do not multiply the coefficents that you need to balance to the Eo cell; you just have to see that the copper is being oxidized, so the sign changes (0.34 → –0.34) and the Cr is being reduced so the sign doesn't change. Then, just add the Eo cells: –0.74 + (–0.34) = –1.08.
← Didn't Know|Knew It →
The standard reduction potentials for certain metals are listed below:
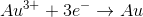
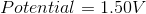

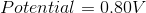

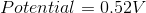




What is the standard potential of a cell in which copper is reduced by iron?
The standard reduction potentials for certain metals are listed below:
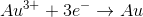




What is the standard potential of a cell in which copper is reduced by iron?
Tap to reveal answer
In order to find the potential of a galvanic cell in which copper is reduced by iron, we need to combine the two half reactions for the metals in question.
Since copper is reduced in the net ionic equation, we can use the reduction potential seen in the table.


Iron must be oxidized in order to reduce the copper. As a result, we must use the reverse reaction in the table. Remember that the table only gives reduction potentials; you will frequently need to charge the reaction to find an oxidation potential.


Notice how the potential for the half reaction has been switched as well. Combining these two reactions gives the following balanced reaction, once the electron transfer is balanced.

Reduction potentials are intensive properties, so we do not multiply the potential of the copper half reaction by two. The total potential is simply the addition of both half reaction potentials.

In order to find the potential of a galvanic cell in which copper is reduced by iron, we need to combine the two half reactions for the metals in question.
Since copper is reduced in the net ionic equation, we can use the reduction potential seen in the table.

Iron must be oxidized in order to reduce the copper. As a result, we must use the reverse reaction in the table. Remember that the table only gives reduction potentials; you will frequently need to charge the reaction to find an oxidation potential.

Notice how the potential for the half reaction has been switched as well. Combining these two reactions gives the following balanced reaction, once the electron transfer is balanced.
Reduction potentials are intensive properties, so we do not multiply the potential of the copper half reaction by two. The total potential is simply the addition of both half reaction potentials.
← Didn't Know|Knew It →
The standard reduction potentials for some metals are listed below:










Which of the following metals is the strongest reducing agent?
The standard reduction potentials for some metals are listed below:





Which of the following metals is the strongest reducing agent?
Tap to reveal answer
The reducing agent is the metal that will be oxidized in the reaction. Since all of the half reactions shown are reduction potentials, we need to switch the half reactions in order to determine which results in the greatest cell potential when the metal is oxidized. Iron will result in a cell potential of 0.44 volts when oxidized, which makes it the strongest reducing agent on the list.
The reducing agent is the metal that will be oxidized in the reaction. Since all of the half reactions shown are reduction potentials, we need to switch the half reactions in order to determine which results in the greatest cell potential when the metal is oxidized. Iron will result in a cell potential of 0.44 volts when oxidized, which makes it the strongest reducing agent on the list.
← Didn't Know|Knew It →
Standard Reduction Potentials
Cr3+(aq) + 3e– → Cr (s) –0.74 V
Cu2+(aq) +2e– → Cu (s) 0.34 V
Consider the following reaction
Cu(s) + Cr3+(aq) ⇌ Cu2+(aq) + Cr(s)
What is the Eo Cell for the reaction?
Standard Reduction Potentials
Cr3+(aq) + 3e– → Cr (s) –0.74 V
Cu2+(aq) +2e– → Cu (s) 0.34 V
Consider the following reaction
Cu(s) + Cr3+(aq) ⇌ Cu2+(aq) + Cr(s)
What is the Eo Cell for the reaction?
Tap to reveal answer
You do not multiply the coefficents that you need to balance to the Eo cell; you just have to see that the copper is being oxidized, so the sign changes (0.34 → –0.34) and the Cr is being reduced so the sign doesn't change. Then, just add the Eo cells: –0.74 + (–0.34) = –1.08.
You do not multiply the coefficents that you need to balance to the Eo cell; you just have to see that the copper is being oxidized, so the sign changes (0.34 → –0.34) and the Cr is being reduced so the sign doesn't change. Then, just add the Eo cells: –0.74 + (–0.34) = –1.08.
← Didn't Know|Knew It →
The standard reduction potentials for certain metals are listed below:
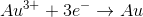
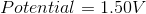

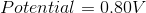

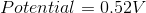




What is the standard potential of a cell in which copper is reduced by iron?
The standard reduction potentials for certain metals are listed below:
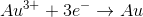




What is the standard potential of a cell in which copper is reduced by iron?
Tap to reveal answer
In order to find the potential of a galvanic cell in which copper is reduced by iron, we need to combine the two half reactions for the metals in question.
Since copper is reduced in the net ionic equation, we can use the reduction potential seen in the table.


Iron must be oxidized in order to reduce the copper. As a result, we must use the reverse reaction in the table. Remember that the table only gives reduction potentials; you will frequently need to charge the reaction to find an oxidation potential.


Notice how the potential for the half reaction has been switched as well. Combining these two reactions gives the following balanced reaction, once the electron transfer is balanced.

Reduction potentials are intensive properties, so we do not multiply the potential of the copper half reaction by two. The total potential is simply the addition of both half reaction potentials.

In order to find the potential of a galvanic cell in which copper is reduced by iron, we need to combine the two half reactions for the metals in question.
Since copper is reduced in the net ionic equation, we can use the reduction potential seen in the table.

Iron must be oxidized in order to reduce the copper. As a result, we must use the reverse reaction in the table. Remember that the table only gives reduction potentials; you will frequently need to charge the reaction to find an oxidation potential.

Notice how the potential for the half reaction has been switched as well. Combining these two reactions gives the following balanced reaction, once the electron transfer is balanced.
Reduction potentials are intensive properties, so we do not multiply the potential of the copper half reaction by two. The total potential is simply the addition of both half reaction potentials.
← Didn't Know|Knew It →
The standard reduction potentials for some metals are listed below:










Which of the following metals is the strongest reducing agent?
The standard reduction potentials for some metals are listed below:





Which of the following metals is the strongest reducing agent?
Tap to reveal answer
The reducing agent is the metal that will be oxidized in the reaction. Since all of the half reactions shown are reduction potentials, we need to switch the half reactions in order to determine which results in the greatest cell potential when the metal is oxidized. Iron will result in a cell potential of 0.44 volts when oxidized, which makes it the strongest reducing agent on the list.
The reducing agent is the metal that will be oxidized in the reaction. Since all of the half reactions shown are reduction potentials, we need to switch the half reactions in order to determine which results in the greatest cell potential when the metal is oxidized. Iron will result in a cell potential of 0.44 volts when oxidized, which makes it the strongest reducing agent on the list.
← Didn't Know|Knew It →
Standard Reduction Potentials
Cr3+(aq) + 3e– → Cr (s) –0.74 V
Cu2+(aq) +2e– → Cu (s) 0.34 V
Consider the following reaction
Cu(s) + Cr3+(aq) ⇌ Cu2+(aq) + Cr(s)
What is the Eo Cell for the reaction?
Standard Reduction Potentials
Cr3+(aq) + 3e– → Cr (s) –0.74 V
Cu2+(aq) +2e– → Cu (s) 0.34 V
Consider the following reaction
Cu(s) + Cr3+(aq) ⇌ Cu2+(aq) + Cr(s)
What is the Eo Cell for the reaction?
Tap to reveal answer
You do not multiply the coefficents that you need to balance to the Eo cell; you just have to see that the copper is being oxidized, so the sign changes (0.34 → –0.34) and the Cr is being reduced so the sign doesn't change. Then, just add the Eo cells: –0.74 + (–0.34) = –1.08.
You do not multiply the coefficents that you need to balance to the Eo cell; you just have to see that the copper is being oxidized, so the sign changes (0.34 → –0.34) and the Cr is being reduced so the sign doesn't change. Then, just add the Eo cells: –0.74 + (–0.34) = –1.08.
← Didn't Know|Knew It →
The standard reduction potentials for certain metals are listed below:
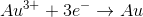
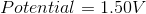

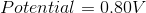

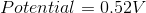




What is the standard potential of a cell in which copper is reduced by iron?
The standard reduction potentials for certain metals are listed below:
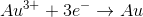




What is the standard potential of a cell in which copper is reduced by iron?
Tap to reveal answer
In order to find the potential of a galvanic cell in which copper is reduced by iron, we need to combine the two half reactions for the metals in question.
Since copper is reduced in the net ionic equation, we can use the reduction potential seen in the table.


Iron must be oxidized in order to reduce the copper. As a result, we must use the reverse reaction in the table. Remember that the table only gives reduction potentials; you will frequently need to charge the reaction to find an oxidation potential.


Notice how the potential for the half reaction has been switched as well. Combining these two reactions gives the following balanced reaction, once the electron transfer is balanced.

Reduction potentials are intensive properties, so we do not multiply the potential of the copper half reaction by two. The total potential is simply the addition of both half reaction potentials.

In order to find the potential of a galvanic cell in which copper is reduced by iron, we need to combine the two half reactions for the metals in question.
Since copper is reduced in the net ionic equation, we can use the reduction potential seen in the table.

Iron must be oxidized in order to reduce the copper. As a result, we must use the reverse reaction in the table. Remember that the table only gives reduction potentials; you will frequently need to charge the reaction to find an oxidation potential.

Notice how the potential for the half reaction has been switched as well. Combining these two reactions gives the following balanced reaction, once the electron transfer is balanced.
Reduction potentials are intensive properties, so we do not multiply the potential of the copper half reaction by two. The total potential is simply the addition of both half reaction potentials.
← Didn't Know|Knew It →
The standard reduction potentials for some metals are listed below:










Which of the following metals is the strongest reducing agent?
The standard reduction potentials for some metals are listed below:





Which of the following metals is the strongest reducing agent?
Tap to reveal answer
The reducing agent is the metal that will be oxidized in the reaction. Since all of the half reactions shown are reduction potentials, we need to switch the half reactions in order to determine which results in the greatest cell potential when the metal is oxidized. Iron will result in a cell potential of 0.44 volts when oxidized, which makes it the strongest reducing agent on the list.
The reducing agent is the metal that will be oxidized in the reaction. Since all of the half reactions shown are reduction potentials, we need to switch the half reactions in order to determine which results in the greatest cell potential when the metal is oxidized. Iron will result in a cell potential of 0.44 volts when oxidized, which makes it the strongest reducing agent on the list.
← Didn't Know|Knew It →
Standard Reduction Potentials
Cr3+(aq) + 3e– → Cr (s) –0.74 V
Cu2+(aq) +2e– → Cu (s) 0.34 V
Consider the following reaction
Cu(s) + Cr3+(aq) ⇌ Cu2+(aq) + Cr(s)
What is the Eo Cell for the reaction?
Standard Reduction Potentials
Cr3+(aq) + 3e– → Cr (s) –0.74 V
Cu2+(aq) +2e– → Cu (s) 0.34 V
Consider the following reaction
Cu(s) + Cr3+(aq) ⇌ Cu2+(aq) + Cr(s)
What is the Eo Cell for the reaction?
Tap to reveal answer
You do not multiply the coefficents that you need to balance to the Eo cell; you just have to see that the copper is being oxidized, so the sign changes (0.34 → –0.34) and the Cr is being reduced so the sign doesn't change. Then, just add the Eo cells: –0.74 + (–0.34) = –1.08.
You do not multiply the coefficents that you need to balance to the Eo cell; you just have to see that the copper is being oxidized, so the sign changes (0.34 → –0.34) and the Cr is being reduced so the sign doesn't change. Then, just add the Eo cells: –0.74 + (–0.34) = –1.08.
← Didn't Know|Knew It →
The standard reduction potentials for certain metals are listed below:
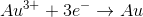
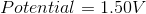

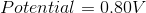

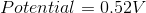




What is the standard potential of a cell in which copper is reduced by iron?
The standard reduction potentials for certain metals are listed below:
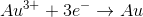




What is the standard potential of a cell in which copper is reduced by iron?
Tap to reveal answer
In order to find the potential of a galvanic cell in which copper is reduced by iron, we need to combine the two half reactions for the metals in question.
Since copper is reduced in the net ionic equation, we can use the reduction potential seen in the table.


Iron must be oxidized in order to reduce the copper. As a result, we must use the reverse reaction in the table. Remember that the table only gives reduction potentials; you will frequently need to charge the reaction to find an oxidation potential.


Notice how the potential for the half reaction has been switched as well. Combining these two reactions gives the following balanced reaction, once the electron transfer is balanced.

Reduction potentials are intensive properties, so we do not multiply the potential of the copper half reaction by two. The total potential is simply the addition of both half reaction potentials.

In order to find the potential of a galvanic cell in which copper is reduced by iron, we need to combine the two half reactions for the metals in question.
Since copper is reduced in the net ionic equation, we can use the reduction potential seen in the table.

Iron must be oxidized in order to reduce the copper. As a result, we must use the reverse reaction in the table. Remember that the table only gives reduction potentials; you will frequently need to charge the reaction to find an oxidation potential.

Notice how the potential for the half reaction has been switched as well. Combining these two reactions gives the following balanced reaction, once the electron transfer is balanced.
Reduction potentials are intensive properties, so we do not multiply the potential of the copper half reaction by two. The total potential is simply the addition of both half reaction potentials.
← Didn't Know|Knew It →
The standard reduction potentials for some metals are listed below:










Which of the following metals is the strongest reducing agent?
The standard reduction potentials for some metals are listed below:





Which of the following metals is the strongest reducing agent?
Tap to reveal answer
The reducing agent is the metal that will be oxidized in the reaction. Since all of the half reactions shown are reduction potentials, we need to switch the half reactions in order to determine which results in the greatest cell potential when the metal is oxidized. Iron will result in a cell potential of 0.44 volts when oxidized, which makes it the strongest reducing agent on the list.
The reducing agent is the metal that will be oxidized in the reaction. Since all of the half reactions shown are reduction potentials, we need to switch the half reactions in order to determine which results in the greatest cell potential when the metal is oxidized. Iron will result in a cell potential of 0.44 volts when oxidized, which makes it the strongest reducing agent on the list.
← Didn't Know|Knew It →
Standard Reduction Potentials
Cr3+(aq) + 3e– → Cr (s) –0.74 V
Cu2+(aq) +2e– → Cu (s) 0.34 V
Consider the following reaction
Cu(s) + Cr3+(aq) ⇌ Cu2+(aq) + Cr(s)
What is the Eo Cell for the reaction?
Standard Reduction Potentials
Cr3+(aq) + 3e– → Cr (s) –0.74 V
Cu2+(aq) +2e– → Cu (s) 0.34 V
Consider the following reaction
Cu(s) + Cr3+(aq) ⇌ Cu2+(aq) + Cr(s)
What is the Eo Cell for the reaction?
Tap to reveal answer
You do not multiply the coefficents that you need to balance to the Eo cell; you just have to see that the copper is being oxidized, so the sign changes (0.34 → –0.34) and the Cr is being reduced so the sign doesn't change. Then, just add the Eo cells: –0.74 + (–0.34) = –1.08.
You do not multiply the coefficents that you need to balance to the Eo cell; you just have to see that the copper is being oxidized, so the sign changes (0.34 → –0.34) and the Cr is being reduced so the sign doesn't change. Then, just add the Eo cells: –0.74 + (–0.34) = –1.08.
← Didn't Know|Knew It →
The standard reduction potentials for certain metals are listed below:
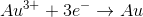
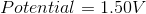

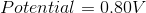

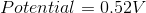




What is the standard potential of a cell in which copper is reduced by iron?
The standard reduction potentials for certain metals are listed below:
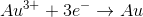




What is the standard potential of a cell in which copper is reduced by iron?
Tap to reveal answer
In order to find the potential of a galvanic cell in which copper is reduced by iron, we need to combine the two half reactions for the metals in question.
Since copper is reduced in the net ionic equation, we can use the reduction potential seen in the table.


Iron must be oxidized in order to reduce the copper. As a result, we must use the reverse reaction in the table. Remember that the table only gives reduction potentials; you will frequently need to charge the reaction to find an oxidation potential.


Notice how the potential for the half reaction has been switched as well. Combining these two reactions gives the following balanced reaction, once the electron transfer is balanced.

Reduction potentials are intensive properties, so we do not multiply the potential of the copper half reaction by two. The total potential is simply the addition of both half reaction potentials.

In order to find the potential of a galvanic cell in which copper is reduced by iron, we need to combine the two half reactions for the metals in question.
Since copper is reduced in the net ionic equation, we can use the reduction potential seen in the table.

Iron must be oxidized in order to reduce the copper. As a result, we must use the reverse reaction in the table. Remember that the table only gives reduction potentials; you will frequently need to charge the reaction to find an oxidation potential.

Notice how the potential for the half reaction has been switched as well. Combining these two reactions gives the following balanced reaction, once the electron transfer is balanced.
Reduction potentials are intensive properties, so we do not multiply the potential of the copper half reaction by two. The total potential is simply the addition of both half reaction potentials.
← Didn't Know|Knew It →