Enthalpy - AP Chemistry
Card 1 of 182
H2O ice melts to liquid H2O \[Heat is added\]
Which of the following is correct?
I. Δ H is positive
II. ΔS is positive
III. ΔH is negative
IV. ΔS is negative
H2O ice melts to liquid H2O \[Heat is added\]
Which of the following is correct?
I. Δ H is positive
II. ΔS is positive
III. ΔH is negative
IV. ΔS is negative
Tap to reveal answer
When heat is added, enthalpy increases, ΔH refers to enthalpy
ΔS refers to entropy, which is disorder; disorder increases as it moves from solid to liquid to gas state.
As ice goes to liquid water, entropy increases
When heat is added, enthalpy increases, ΔH refers to enthalpy
ΔS refers to entropy, which is disorder; disorder increases as it moves from solid to liquid to gas state.
As ice goes to liquid water, entropy increases
← Didn't Know|Knew It →
Calculate ΔH for the following reaction:
CH4 (g) + O2 (g) ⇌ CO2 (g) + H2O (l)
Compound ΔH
CH4 (g) -74.8 kJ/mol
H2O (l) -285.8 kJ/mol
CO2 (g) -393.5 kJ/mol
Calculate ΔH for the following reaction:
CH4 (g) + O2 (g) ⇌ CO2 (g) + H2O (l)
Compound ΔH
CH4 (g) -74.8 kJ/mol
H2O (l) -285.8 kJ/mol
CO2 (g) -393.5 kJ/mol
Tap to reveal answer
ΔH = Σ ΔHf products - Σ ΔHf reactants
ΔHf O2 or any element is 0
First step is to balance the equation:
CH4 (g) + O2 (g) ⇌ CO2 (g) + 2H2O (l)
ΔH = Σ ΔHf products - Σ ΔHf reactants
= \[-393.5 kJ/mol + 2(-285.8) kJ/mol\] - (-74.8 kJ/mol)
= -890.3 kJ/mol
ΔH = Σ ΔHf products - Σ ΔHf reactants
ΔHf O2 or any element is 0
First step is to balance the equation:
CH4 (g) + O2 (g) ⇌ CO2 (g) + 2H2O (l)
ΔH = Σ ΔHf products - Σ ΔHf reactants
= \[-393.5 kJ/mol + 2(-285.8) kJ/mol\] - (-74.8 kJ/mol)
= -890.3 kJ/mol
← Didn't Know|Knew It →
Which of the following is a true statement regarding the energy involved in the formation of Methane, CH4, from graphite C(s) and H2(g) ?
Which of the following is a true statement regarding the energy involved in the formation of Methane, CH4, from graphite C(s) and H2(g) ?
Tap to reveal answer
This problem requires a knowledge of the energy involved when bonds are broken versus when bonds are formed. Energy is released when bonds are formed, and energy is consumed when bonds are broken. Here, methane is being formed from C and H2. Since the carbon source, graphite, is monoatomic the carbon will be involved only in the formation of C–H bonds on it's way to forming methane. The H however exists as H2 so bonds will need to be broken to allow for C–H bonds to form. So first energy is consumed to break the H–H bonds, it is then released when the C–H bonds form.
This problem requires a knowledge of the energy involved when bonds are broken versus when bonds are formed. Energy is released when bonds are formed, and energy is consumed when bonds are broken. Here, methane is being formed from C and H2. Since the carbon source, graphite, is monoatomic the carbon will be involved only in the formation of C–H bonds on it's way to forming methane. The H however exists as H2 so bonds will need to be broken to allow for C–H bonds to form. So first energy is consumed to break the H–H bonds, it is then released when the C–H bonds form.
← Didn't Know|Knew It →
The formation of nitrogen dioxide is a two step process.




The net reaction is  .
.
What is the change in enthalpy when creating four moles of nitrogen dioxide?
The formation of nitrogen dioxide is a two step process.


The net reaction is 
What is the change in enthalpy when creating four moles of nitrogen dioxide?
Tap to reveal answer
Hess's law states that the change in enthalpy for a total reaction can be considered equal to the sum of the enthalpy changes for every step involved in the reaction. In other words, we can determine the enthalpy change for nitrogen dioxide by adding the enthalpy changes for both steps involved in its formation.


This gives us the total change in enthalpy for the listed reaction,  . Because the question asks for the enthalpy change for four moles of nitrogen dioxide, the value must be doubled. The reaction only produces two moles of nitrogen dioxide.
. Because the question asks for the enthalpy change for four moles of nitrogen dioxide, the value must be doubled. The reaction only produces two moles of nitrogen dioxide.


Hess's law states that the change in enthalpy for a total reaction can be considered equal to the sum of the enthalpy changes for every step involved in the reaction. In other words, we can determine the enthalpy change for nitrogen dioxide by adding the enthalpy changes for both steps involved in its formation.
This gives us the total change in enthalpy for the listed reaction, 
← Didn't Know|Knew It →
Which of the following statements is true concerning a chemical reaction?
Which of the following statements is true concerning a chemical reaction?
Tap to reveal answer
When a chemical reaction is represented graphically, we see that the enthalpy change is reversed between the forward and reverse reactions. If a reaction produces energy in a forward process, it will require an input of energy in the reverse process, and vice versa.


A catalyst only affects the rate of a chemical reaction; it does not affect the equilibrium. Finally, exothermic reactions are not always spontaneous, but will have lower activation of energies compared to endothermic reactions.
When a chemical reaction is represented graphically, we see that the enthalpy change is reversed between the forward and reverse reactions. If a reaction produces energy in a forward process, it will require an input of energy in the reverse process, and vice versa.
A catalyst only affects the rate of a chemical reaction; it does not affect the equilibrium. Finally, exothermic reactions are not always spontaneous, but will have lower activation of energies compared to endothermic reactions.
← Didn't Know|Knew It →






What is the change in enthalpy for the given reaction?




What is the change in enthalpy for the given reaction?
Tap to reveal answer
The change in enthalpy is calculated by:

When  cannot be measured, it can be calculated from known reactions. In this case the known reactions are given.
cannot be measured, it can be calculated from known reactions. In this case the known reactions are given.






Since the reactions are in the correct order, adding all the  values together can be used to calculate the
values together can be used to calculate the  of the reaction.
of the reaction.



The change in enthalpy is calculated by:
When 



Since the reactions are in the correct order, adding all the 

← Didn't Know|Knew It →






What is the enthalpy of the following reaction?




What is the enthalpy of the following reaction?
Tap to reveal answer
The change in enthalpy is calculated by:

When  cannot be measured, it can be calculated from known enthalpies of formation.
cannot be measured, it can be calculated from known enthalpies of formation.






It is important to first balance the reaction before performing calculations. The coefficients are important in determining the change in enthalpy of a reaction. For this particular reaction, since there are two moles of product, the enthalpy of formation for  must be multiplied by two.
must be multiplied by two.




The change in enthalpy is calculated by:
When 



It is important to first balance the reaction before performing calculations. The coefficients are important in determining the change in enthalpy of a reaction. For this particular reaction, since there are two moles of product, the enthalpy of formation for 
← Didn't Know|Knew It →






What is the change in enthalpy for the following reaction?




What is the change in enthalpy for the following reaction?
Tap to reveal answer
The change in enthalpy is calculated by:

When  cannot be measured, it can be calculated from known enthalpies of formation.
cannot be measured, it can be calculated from known enthalpies of formation.






It is important to first balance the reaction before performing calculations. The coefficients are important in determining the change in enthalpy of a reaction.




The change in enthalpy is calculated by:
When 



It is important to first balance the reaction before performing calculations. The coefficients are important in determining the change in enthalpy of a reaction.
← Didn't Know|Knew It →






What is the change in enthalpy for the following reaction?




What is the change in enthalpy for the following reaction?
Tap to reveal answer
The change in enthalpy is calculated by:

When  cannot be measured, it can be calculated from known enthalpies of formation.
cannot be measured, it can be calculated from known enthalpies of formation.






It is important to first balance the reaction before performing calculations. The coefficients are important in determining the change in enthalpy of a reaction.




The change in enthalpy is calculated by:
When 



It is important to first balance the reaction before performing calculations. The coefficients are important in determining the change in enthalpy of a reaction.
← Didn't Know|Knew It →
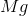



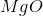

What is the change in enthalpy for the following reaction?

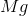

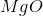
What is the change in enthalpy for the following reaction?
Tap to reveal answer
The change in enthalpy is calculated by:

When  cannot be measured, it can be calculated from known enthalpies of formation.
cannot be measured, it can be calculated from known enthalpies of formation.
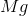



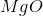

It is important to first balance the reaction before performing calculations. The coefficients are important in determining the change in enthalpy of a reaction.




The change in enthalpy is calculated by:
When 
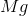

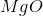
It is important to first balance the reaction before performing calculations. The coefficients are important in determining the change in enthalpy of a reaction.
← Didn't Know|Knew It →








What is the change in enthalpy for the following reaction?





What is the change in enthalpy for the following reaction?
Tap to reveal answer
The change in enthalpy is calculated by:

When  cannot be measured, it can be calculated from known enthalpies of formation.
cannot be measured, it can be calculated from known enthalpies of formation.








It is important to first balance the reaction before performing calculations. The coefficients are important in determining the change in enthalpy of a reaction.




The change in enthalpy is calculated by:
When 




It is important to first balance the reaction before performing calculations. The coefficients are important in determining the change in enthalpy of a reaction.
← Didn't Know|Knew It →
Consider the following combustion reaction:

The following list is the enthalpies of formation for the compounds:



If one mole of propane is burned, what is the enthalpy of the reaction?
Consider the following combustion reaction:
The following list is the enthalpies of formation for the compounds:
If one mole of propane is burned, what is the enthalpy of the reaction?
Tap to reveal answer
Since we have the enthalpies of formation, we can find the enthalpy of the reaction using the following equation:

We will need to use the coefficients from the balanced equation to calculate the enthalpy.

Since oxygen gas is elemental, it does not have an enthalpy of formation, and is omitted from the equation.



Since we have the enthalpies of formation, we can find the enthalpy of the reaction using the following equation:
We will need to use the coefficients from the balanced equation to calculate the enthalpy.
Since oxygen gas is elemental, it does not have an enthalpy of formation, and is omitted from the equation.
← Didn't Know|Knew It →
Which of the following equations is correct for finding change in enthalpy?
Which of the following equations is correct for finding change in enthalpy?
Tap to reveal answer
The change in enthalpy of a reaction is determined by the amount of heat gained when the products bonds form, versus the amount of heat lost to break the bonds in the reactants. The correct formula for the enthalpy of reaction is the difference between the enthalpy of the products and that of the reactants:

The change in enthalpy of a reaction is determined by the amount of heat gained when the products bonds form, versus the amount of heat lost to break the bonds in the reactants. The correct formula for the enthalpy of reaction is the difference between the enthalpy of the products and that of the reactants:
← Didn't Know|Knew It →




Based on the given reactions, what is the enthalpy change in the following reaction?



Based on the given reactions, what is the enthalpy change in the following reaction?
Tap to reveal answer
The reactions given show the heats of formation of all the reactants and products, and we know the heat of formation of  is zero because it is a pure element. Formation of the product,
is zero because it is a pure element. Formation of the product,  gives us
gives us  . For the reactants,
. For the reactants,  gives us
gives us  plus
plus  for
for  . Recall that the overall reaction's change in enthalpy is:
. Recall that the overall reaction's change in enthalpy is:

Thus for this overall reaction:

The reactions given show the heats of formation of all the reactants and products, and we know the heat of formation of 






Thus for this overall reaction:
← Didn't Know|Knew It →
H2O ice melts to liquid H2O \[Heat is added\]
Which of the following is correct?
I. Δ H is positive
II. ΔS is positive
III. ΔH is negative
IV. ΔS is negative
H2O ice melts to liquid H2O \[Heat is added\]
Which of the following is correct?
I. Δ H is positive
II. ΔS is positive
III. ΔH is negative
IV. ΔS is negative
Tap to reveal answer
When heat is added, enthalpy increases, ΔH refers to enthalpy
ΔS refers to entropy, which is disorder; disorder increases as it moves from solid to liquid to gas state.
As ice goes to liquid water, entropy increases
When heat is added, enthalpy increases, ΔH refers to enthalpy
ΔS refers to entropy, which is disorder; disorder increases as it moves from solid to liquid to gas state.
As ice goes to liquid water, entropy increases
← Didn't Know|Knew It →
Calculate ΔH for the following reaction:
CH4 (g) + O2 (g) ⇌ CO2 (g) + H2O (l)
Compound ΔH
CH4 (g) -74.8 kJ/mol
H2O (l) -285.8 kJ/mol
CO2 (g) -393.5 kJ/mol
Calculate ΔH for the following reaction:
CH4 (g) + O2 (g) ⇌ CO2 (g) + H2O (l)
Compound ΔH
CH4 (g) -74.8 kJ/mol
H2O (l) -285.8 kJ/mol
CO2 (g) -393.5 kJ/mol
Tap to reveal answer
ΔH = Σ ΔHf products - Σ ΔHf reactants
ΔHf O2 or any element is 0
First step is to balance the equation:
CH4 (g) + O2 (g) ⇌ CO2 (g) + 2H2O (l)
ΔH = Σ ΔHf products - Σ ΔHf reactants
= \[-393.5 kJ/mol + 2(-285.8) kJ/mol\] - (-74.8 kJ/mol)
= -890.3 kJ/mol
ΔH = Σ ΔHf products - Σ ΔHf reactants
ΔHf O2 or any element is 0
First step is to balance the equation:
CH4 (g) + O2 (g) ⇌ CO2 (g) + 2H2O (l)
ΔH = Σ ΔHf products - Σ ΔHf reactants
= \[-393.5 kJ/mol + 2(-285.8) kJ/mol\] - (-74.8 kJ/mol)
= -890.3 kJ/mol
← Didn't Know|Knew It →
Which of the following is a true statement regarding the energy involved in the formation of Methane, CH4, from graphite C(s) and H2(g) ?
Which of the following is a true statement regarding the energy involved in the formation of Methane, CH4, from graphite C(s) and H2(g) ?
Tap to reveal answer
This problem requires a knowledge of the energy involved when bonds are broken versus when bonds are formed. Energy is released when bonds are formed, and energy is consumed when bonds are broken. Here, methane is being formed from C and H2. Since the carbon source, graphite, is monoatomic the carbon will be involved only in the formation of C–H bonds on it's way to forming methane. The H however exists as H2 so bonds will need to be broken to allow for C–H bonds to form. So first energy is consumed to break the H–H bonds, it is then released when the C–H bonds form.
This problem requires a knowledge of the energy involved when bonds are broken versus when bonds are formed. Energy is released when bonds are formed, and energy is consumed when bonds are broken. Here, methane is being formed from C and H2. Since the carbon source, graphite, is monoatomic the carbon will be involved only in the formation of C–H bonds on it's way to forming methane. The H however exists as H2 so bonds will need to be broken to allow for C–H bonds to form. So first energy is consumed to break the H–H bonds, it is then released when the C–H bonds form.
← Didn't Know|Knew It →
The formation of nitrogen dioxide is a two step process.




The net reaction is  .
.
What is the change in enthalpy when creating four moles of nitrogen dioxide?
The formation of nitrogen dioxide is a two step process.


The net reaction is 
What is the change in enthalpy when creating four moles of nitrogen dioxide?
Tap to reveal answer
Hess's law states that the change in enthalpy for a total reaction can be considered equal to the sum of the enthalpy changes for every step involved in the reaction. In other words, we can determine the enthalpy change for nitrogen dioxide by adding the enthalpy changes for both steps involved in its formation.


This gives us the total change in enthalpy for the listed reaction,  . Because the question asks for the enthalpy change for four moles of nitrogen dioxide, the value must be doubled. The reaction only produces two moles of nitrogen dioxide.
. Because the question asks for the enthalpy change for four moles of nitrogen dioxide, the value must be doubled. The reaction only produces two moles of nitrogen dioxide.


Hess's law states that the change in enthalpy for a total reaction can be considered equal to the sum of the enthalpy changes for every step involved in the reaction. In other words, we can determine the enthalpy change for nitrogen dioxide by adding the enthalpy changes for both steps involved in its formation.
This gives us the total change in enthalpy for the listed reaction, 
← Didn't Know|Knew It →
Which of the following statements is true concerning a chemical reaction?
Which of the following statements is true concerning a chemical reaction?
Tap to reveal answer
When a chemical reaction is represented graphically, we see that the enthalpy change is reversed between the forward and reverse reactions. If a reaction produces energy in a forward process, it will require an input of energy in the reverse process, and vice versa.


A catalyst only affects the rate of a chemical reaction; it does not affect the equilibrium. Finally, exothermic reactions are not always spontaneous, but will have lower activation of energies compared to endothermic reactions.
When a chemical reaction is represented graphically, we see that the enthalpy change is reversed between the forward and reverse reactions. If a reaction produces energy in a forward process, it will require an input of energy in the reverse process, and vice versa.
A catalyst only affects the rate of a chemical reaction; it does not affect the equilibrium. Finally, exothermic reactions are not always spontaneous, but will have lower activation of energies compared to endothermic reactions.
← Didn't Know|Knew It →






What is the change in enthalpy for the given reaction?




What is the change in enthalpy for the given reaction?
Tap to reveal answer
The change in enthalpy is calculated by:

When  cannot be measured, it can be calculated from known reactions. In this case the known reactions are given.
cannot be measured, it can be calculated from known reactions. In this case the known reactions are given.






Since the reactions are in the correct order, adding all the  values together can be used to calculate the
values together can be used to calculate the  of the reaction.
of the reaction.



The change in enthalpy is calculated by:
When 



Since the reactions are in the correct order, adding all the 

← Didn't Know|Knew It →