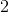pH
Help Questions
SAT Subject Test in Chemistry › pH
Questions 1 - 1
1
A scientist makes a solution by adding 0.2 grams of 
Explanation






AI TutorYour personal study buddy
Question of the DayDaily practice to build your skills
FlashcardsStudy and memorize key concepts
WorksheetsBuild custom practice quizzes
AI SolverStep-by-step problem solutions
Learn by ConceptMaster concepts step by step
Diagnostic TestsAssess your knowledge level
Practice TestsTest your skills with exam questions
GamesLearn through free games