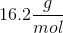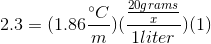Freezing Point
Help Questions
Physical Chemistry › Freezing Point
Questions 1 - 1
1
When 20 grams of a solute is added to a liter of water, the freezing point becomes 
Assume that the solute does not make ions in solution.
Explanation
We can use the freezing point depression equation in order to determine the molar mass of the unknown solute.
AI TutorYour personal study buddy
Question of the DayDaily practice to build your skills
FlashcardsStudy and memorize key concepts
AI SolverStep-by-step problem solutions
Learn by ConceptMaster concepts step by step
Practice TestsTest your skills with exam questions
QuizzesTest your knowledge with a quiz
GamesLearn through free games