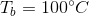Boiling Point
Help Questions
Physical Chemistry › Boiling Point
Questions 1 - 1
1
What is the boiling point of a 100mL solution of water after the addition of 29g of sodium chloride?
Explanation
We can use the boiling point elevation equation in order to find the new boiling point for the solution.
The change in temperature will be equal to the boiling point elevation constant multiplied by the molality of the solution multiplied by the van't Hoff factor. The van't Hoff factor is a variable that incorporates how many ions the added solute will dissolve into when in solution. Sodium chloride will dissolve into two ions, so the van't Hoff factor is 2.
This means that the boiling point of the water has been elevated by 5.2 degrees, meaning that the boiling point of the solution is 105.2 degrees celsius.
AI TutorYour personal study buddy
Question of the DayDaily practice to build your skills
FlashcardsStudy and memorize key concepts
AI SolverStep-by-step problem solutions
Learn by ConceptMaster concepts step by step
Practice TestsTest your skills with exam questions
QuizzesTest your knowledge with a quiz
GamesLearn through free games