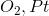Organic Oxidizing Agents
Help Questions
Organic Chemistry › Organic Oxidizing Agents
Which of the following reagents can turn primary alcohols into a carboxylic acid?
Jones Reagent (chromic acid in acetone)
Tollen's test
PCC
Explanation
The Jones reagent can convert primary alcohol to acids and secondary alcohols to ketones. The Tollen's test only converts aldehydes to carboxylic acids. PCC can only convert primary and secondary alcohol to aldehydes and ketones, respectively. 

Which of the following compounds is not a reducing agent?
Explanation

What is an appropriate reagent to convert a primary alcohol to an aldehyde?
Explanation
To form the aldehyde, the alcohol must be oxidized. However, potassium permanganate and chromic acid are too strong and would yield a carboxylic acid. Ozonolysis works with alkenes and oxygen over platinum would not react. PCC is correct because it will oxidize the alcohol to form an aldehyde but is too weak to continue on to form the carboxylic acid.
Which of the following reagents can turn primary alcohols into a carboxylic acid?
Jones Reagent (chromic acid in acetone)
Tollen's test
PCC
Explanation
The Jones reagent can convert primary alcohol to acids and secondary alcohols to ketones. The Tollen's test only converts aldehydes to carboxylic acids. PCC can only convert primary and secondary alcohol to aldehydes and ketones, respectively. 

What is an appropriate reagent to convert a primary alcohol to an aldehyde?
Explanation
To form the aldehyde, the alcohol must be oxidized. However, potassium permanganate and chromic acid are too strong and would yield a carboxylic acid. Ozonolysis works with alkenes and oxygen over platinum would not react. PCC is correct because it will oxidize the alcohol to form an aldehyde but is too weak to continue on to form the carboxylic acid.
Which of the following compounds is not a reducing agent?
Explanation


Which reagent is best-suited to accomplish the given reaction?
PCC
Explanation
PCC is an oxidizing agent that reacts with primary and secondary alcohols. However, it is less reactive than potassium permanganate and chromic acid. PCC differs from chromic acid by oxidizing primary alcohols to aldehydes, whereas chromic acid oxidizes primary alcohols and aldehydes to carboxylic acids. The desired product of the reaction given requires that the primary alcohol be oxidized to an aldehyde, so PCC is the best option. 
What is the product of the reaction shown?
Explanation
PCC can be used to oxidize primary alcohols into aldehydes, or secondary alcohols into ketones. The starting material shown is a secondary alcohol, so the product will be a ketone (a carbonyl (
What is the product of 1-pentanol when it is treated with PCC?
Pentanal
1-pentanone
2-pentanol
No reaction occurs
None of these
Explanation
PCC is an oxidizing agent. It converts alcohols to carbonyls, but is not strong enough to convert a primary alcohol into a carboxylic acid. It only converts primary alcohols to aldehydes, and secondary alcohols to ketones. 1-pentanol is a primary alcohol so it will be converted to the aldehyde pentanal.
What is the product of the reaction shown?
Explanation
PCC can be used to oxidize primary alcohols into aldehydes, or secondary alcohols into ketones. The starting material shown is a secondary alcohol, so the product will be a ketone (a carbonyl (