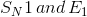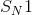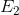Organic Intermediates
Help Questions
Organic Chemistry › Organic Intermediates
Identify the main functional groups in the pictured molecule.
Benzene, imine, aldehyde
Benzene, amide, aldehyde
Phenol, amine, ketone
Phenol, imine, ketone
Explanation
1. Benzene
2. Imine
3. Aldehyde
Identify the main functional groups in the pictured molecule.
Benzene, imine, aldehyde
Benzene, amide, aldehyde
Phenol, amine, ketone
Phenol, imine, ketone
Explanation
1. Benzene
2. Imine
3. Aldehyde
Which of the following carbocation intermediates requires the least activation energy?




Cannot be determined
Explanation
The more stable the carbocation, the lower the activation energy for reaching that intermediate will be. The more substituted a carbocation is, the more stable it is. The carbocation bonded to three alkanes (tertiary carbocation) is the most stable, and thus the correct answer.
Secondary carbocations will require more energy than tertiary, and primary carbocations will require the most energy.
Which of the following carbocation intermediates requires the least activation energy?




Cannot be determined
Explanation
The more stable the carbocation, the lower the activation energy for reaching that intermediate will be. The more substituted a carbocation is, the more stable it is. The carbocation bonded to three alkanes (tertiary carbocation) is the most stable, and thus the correct answer.
Secondary carbocations will require more energy than tertiary, and primary carbocations will require the most energy.
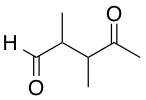
A researcher wants to convert the given molecule's ketone group into a tertiary alcohol. Select the correct order of steps she must take to produce a tertiary alcohol at the ketone, but leave the aldehyde intact.
Ethane-1,2-diol + MeMgBr + H+ and heat
2 MeMgBr + H+
MeMgBr + H+
MeMgBr + H+ + ethane-1,2-diol
Ethane-1,2-diol + H+ and heat + MeMgBr
Explanation
An aldehyde is more electrophilic than a ketone, so to do chemistry on the ketone, we must protect the aldehyde. A common protecting group for aldehydes and ketones is ethane-1,2-diol, as it forms a meta-stable five-membered acetal, which can be hydrolyzed to produce the original aldehyde or ketone by applying heat and acid.
As shown in the scheme below, which corresponds to the correct answer choice, once the aldehyde is protected, then the ketone can be reacted with the Grignard MeMgBr reagent to add a methyl group at the carbonyl. An acid workup removes the protecting group to reveal the original aldehyde, and affords the desired tertiary alcohol.

The schemes below illustrate why each of the other answer choices is wrong, as no other sequence will produce the desired product:

A researcher wants to convert the given molecule's ketone group into a tertiary alcohol. Select the correct order of steps she must take to produce a tertiary alcohol at the ketone, but leave the aldehyde intact.

Ethane-1,2-diol + MeMgBr + H+ and heat
2 MeMgBr + H+
MeMgBr + H+
MeMgBr + H+ + ethane-1,2-diol
Ethane-1,2-diol + H+ and heat + MeMgBr
Explanation
An aldehyde is more electrophilic than a ketone, so to do chemistry on the ketone, we must protect the aldehyde. A common protecting group for aldehydes and ketones is ethane-1,2-diol, as it forms a meta-stable five-membered acetal, which can be hydrolyzed to produce the original aldehyde or ketone by applying heat and acid.
As shown in the scheme below, which corresponds to the correct answer choice, once the aldehyde is protected, then the ketone can be reacted with the Grignard MeMgBr reagent to add a methyl group at the carbonyl. An acid workup removes the protecting group to reveal the original aldehyde, and affords the desired tertiary alcohol.

The schemes below illustrate why each of the other answer choices is wrong, as no other sequence will produce the desired product:

Under which reaction mechanism can rearrangements occur?
Explanation
Rearrangements generally require carbocations. Carbocations are only formed under mechanisms with unimolecular rate-limiting steps (i.e. carbocation formation). 
Under which reaction mechanism can rearrangements occur?
Explanation
Rearrangements generally require carbocations. Carbocations are only formed under mechanisms with unimolecular rate-limiting steps (i.e. carbocation formation). 
Which of the following transformations includes an enolate intermediate?
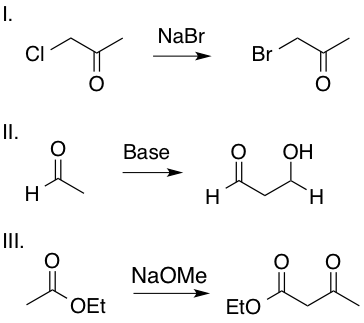
II and III
I and III
I and II
I, II, and III
III only
Explanation
Enolates are formed by an oxygen anion bound to an alkene carbon. Reactions II and III include an enolate intermediate, as shown in the mechanisms below, whereas reaction I is a simple SN2 reaction and does not include an enolate intermediate. Enolates are highlighted in red.

Which of the following transformations includes an enolate intermediate?

II and III
I and III
I and II
I, II, and III
III only
Explanation
Enolates are formed by an oxygen anion bound to an alkene carbon. Reactions II and III include an enolate intermediate, as shown in the mechanisms below, whereas reaction I is a simple SN2 reaction and does not include an enolate intermediate. Enolates are highlighted in red.