Help with Carboxylic Acid Synthesis
Help Questions
Organic Chemistry › Help with Carboxylic Acid Synthesis

What is the product of the given reaction?

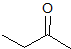



Explanation
The hydrolyzation of a nitrile with hydronium leads to a carboxylic acid. Only one choice is a carboxylic acid.
Please choose the best answer for the following question.
Which of the following reagents is best for converting a primary alcohol to a carboxylic acid?


PCC
Explanation

Which of the following is not a derivative of a carboxylic acid?
Aldehyde
Ester
Amide
All of these are carboxylic acid derivatives
Explanation
Aldehyde is not a derivative of carboxylic acid. Esters can be derived from carboxylic acids by reacting them with 



Which of the following reagents is needed to convert an amide into a carboxylic acid?
All of these
Explanation
As a general rule, any carboxylic acid derivative can be converted into al carboxylic acid when reacting with
Which one of the following compounds can produce a carboxylic acid only when reacted with sodium dichromate and sulfuric acid?
1-pentanol
2-pentanol
2-methylpentan-2-ol
2-pentanone
Pentanal
Explanation
After recognizing the reagents given, we know we need to begin with an alcohol. The alcohol choices differ only by how substituted they are.
For a carboxylic acid to form from a reaction with sodium dichromate and sulfuric acid, a primary alcohol needs to be available. Therefore, 1-pentanol is the correct answer.
When 3-chloroheptane undergoes malonic ester synthesis, the final product is .
3-ethylheptanoic acid
None of the other answers
nonanoic acid
malonic ester
a ten-carbon ketone
Explanation
The malonic ester is deprotonated at the most acidic hydrogen, the one on the carbon between the two oxygens. The electrons from that bond to the hydrogen form a carbon-carbon double bond while electrons from the oxygen-carbon double bond go to the oxygen atom. This is the enolate form. The chlorine leaves the 3-chloroheptane and the electrons from the carbon-carbon double bond bond to the carbon that the chlorine left. At the same time, the carbonyl is reformed. After deesterfication, 3-ethylheptanoic acid is formed.
Which of the following reactions would NOT produce a carboxylic acid?
Explanation
PCC is considered a weak oxidizing agent. The reaction of a primary alcohol with PCC would only yield an aldehyde, while reaction with a secondary alcohol will yield a ketone. PCC will not be used to generate carboxylic acids.
A stronger oxidation, like 












