Help with Aldehyde and Ketone Reactions
Help Questions
Organic Chemistry › Help with Aldehyde and Ketone Reactions

What is the product of the reaction given?




Explanation
Below is the mechanism for the reaction given which is called the McMurry Reaction. It is a titanium (Ti) prompted pinacol coupling followed by deoxygenation.


What is the product of the reaction given?




Explanation
Below is the mechanism for the reaction given which is called the McMurry Reaction. It is a titanium (Ti) prompted pinacol coupling followed by deoxygenation.


In an aprotic solvent, what would be the product of the reaction given?




Explanation
This reaction involves the generation of a radical by single electron transfer that couple through carbon-carbon bond formation to form the product pinacol. This reaction as shown below occurs in an aprotic solvlent:


Which of the labeled hydrogens in the given molecule is the most acidic?
C
A
B
D
Explanation
This question is asking what the most acidic hydrogen is in an aldehyde. To identify the most acidic hydrogen, we'll need to consider which conjugate base will be the most stable after the loss of hydrogen. When the alpha-hydrogen is lost, the resulting carbanion will be the most stabilized due to resonance with the adjacent carbonyl group. This resonance helps to distribute the negative charge on the carbanion over a greater area, which contributes to the greater stability of this conjugate base. Abstraction of a hydrogen from any of the other position would result in a carbanion that could not participate in resonance and thus would not be as stable.

Choose the appropriate starting compound based on the reaction conditions and major product shown in the figure.

IV only
I only
II only
III only
Either I or II
Explanation
In the presence of a strong base and benzene, ethylene glycol will convert ketones to acetals as seen in the product shown above. The given reagents will not react with esters or alcohols. The only given compound containing a ketone is molecule IV, the correct answer. This type of reaction is useful in organic synthesis when reaction at an ester is desired, but a ketone must be protected from reacting simultaneously. The acetal formed is often called a protecting group and may be reversed by addition of acid.

If the given compound is reacted with 





Explanation
LiAlH4 is a very strong reducing agent and it is a reactant that can reduce acyl chlorides into alcohols. There are 2 alcohols to choose from and now it needs to be known that LiAlH4 doesn't add anything but hydrogens to its substrate. (Note that in the correct answer, there is another H that is not drawn on the carbon bearing the hydroxyl group).

What is the product of the given reaction?



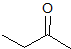
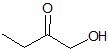
Explanation
This is a Grignard reagent carbon-carbon bond forming reaction. This reaction is being used with an ester which goes through a ketone intermediate with requires a second attack form the organolithium to reduce to an alcohol. Esters and acyl chlorides go to an alcohol with two of the same R groups from the organolithium in a Grignard reaction. This structure fits the bill:
What is the product of the reaction between 2-butanone and lithium aluminum hydride?
2-butanol
3-butanol
3-butanone
Butane
None of these
Explanation
Lithium aluminum hydride is a reducing agent. It reduces ketones and carboxylic acids to alcohols. 2-butanone is a 4-carbon chain with a double bond between an oxygen and carbon 2. The reduction turns the double bond with oxygen into a single bond to a hydroxy group. This makes the product 2-butanol.