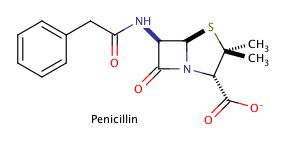
Isomers - Organic Chemistry
Card 1 of 296
The correct answer is three. The key to finding chiral carbons is to look for carbons that are attached to four different substituents. We can immediately eliminate any carbons that are involved in double bonds, or that have two hydrogens attached. Given this, we find that there are three chiral carbons. Note that carbon chains of varying content will qualify as different substituents, allowing chiral carbons to bond to two other carbons.
The correct answer is three. The key to finding chiral carbons is to look for carbons that are attached to four different substituents. We can immediately eliminate any carbons that are involved in double bonds, or that have two hydrogens attached. Given this, we find that there are three chiral carbons. Note that carbon chains of varying content will qualify as different substituents, allowing chiral carbons to bond to two other carbons.
← Didn't Know|Knew It →
How many stereoisomers would be obtained by the hydrogenation of compound C?

How many stereoisomers would be obtained by the hydrogenation of compound C?

Tap to reveal answer
The hydrogenation of compound Cwould add two hydrogen atoms across the double bond, but would generate only one new stereocenter. This stereocenter would be found on the third carbon in the chain (from the right), which would be bound to the phenyl substituent, a methyl group, a hydrogen atom, and the remaining branched carbon chain.
The hydrogenation reaction would create a racemic mixture of both possible orientations of this stereocenter, with both enantiomers present in equal amounts. There would this be two stereoisomer products obtained from the hydrogenation of compound C.
The hydrogenation of compound Cwould add two hydrogen atoms across the double bond, but would generate only one new stereocenter. This stereocenter would be found on the third carbon in the chain (from the right), which would be bound to the phenyl substituent, a methyl group, a hydrogen atom, and the remaining branched carbon chain.
The hydrogenation reaction would create a racemic mixture of both possible orientations of this stereocenter, with both enantiomers present in equal amounts. There would this be two stereoisomer products obtained from the hydrogenation of compound C.
← Didn't Know|Knew It →
How many stereoisomers are possible for the compound 2,3,4-trimethylpentane?
How many stereoisomers are possible for the compound 2,3,4-trimethylpentane?
Tap to reveal answer
2,3,4-trimethylpentane does not contain any stereocenters. The structure is a five-carbon chain, with the end carbons bonded to three hydrogens each. The three central carbons each carry a methyl group and a hydrogen atom.
Remember that a stereocenter is only present when a carbon is bound to four different substituents. The 2 and 4 carbons both have two methyl groups (the end carbons and the added groups), so they would contain a plane of symmetry and would not be stereocenters. Likewise, a plane of symmetry exists at the 3 carbon; the substituents toward the 1 carbon and 5 carbon are the same. This compound, therefore, would have no stereocenters and could only exist as one stereoisomer.

2,3,4-trimethylpentane does not contain any stereocenters. The structure is a five-carbon chain, with the end carbons bonded to three hydrogens each. The three central carbons each carry a methyl group and a hydrogen atom.
Remember that a stereocenter is only present when a carbon is bound to four different substituents. The 2 and 4 carbons both have two methyl groups (the end carbons and the added groups), so they would contain a plane of symmetry and would not be stereocenters. Likewise, a plane of symmetry exists at the 3 carbon; the substituents toward the 1 carbon and 5 carbon are the same. This compound, therefore, would have no stereocenters and could only exist as one stereoisomer.
← Didn't Know|Knew It →

Which two of the molecules shown are enantiomers?

Which two of the molecules shown are enantiomers?
Tap to reveal answer
The enantiomer of a molecule with multiple chiral centers is formed through configurational inversion at every chiral center. By rotating the ring of molecule III 180 degrees about the bond connected to its carbon chain, it is seen that molecules I and III are constitutionally identical with opposite configurations at every chiral center. These compounds are enantiomers. Tip: mental visualization of bond rotations and other transformations is among the most common difficulties experienced in organic chemistry courses. The use of a molecular modeling kit may greatly assist in molecular visualization. Molecules I and II are the same, just rotated 180 degrees.
The enantiomer of a molecule with multiple chiral centers is formed through configurational inversion at every chiral center. By rotating the ring of molecule III 180 degrees about the bond connected to its carbon chain, it is seen that molecules I and III are constitutionally identical with opposite configurations at every chiral center. These compounds are enantiomers. Tip: mental visualization of bond rotations and other transformations is among the most common difficulties experienced in organic chemistry courses. The use of a molecular modeling kit may greatly assist in molecular visualization. Molecules I and II are the same, just rotated 180 degrees.
← Didn't Know|Knew It →
Which of the following best describes an S-enantiomer?
Which of the following best describes an S-enantiomer?
Tap to reveal answer
S configuration deals with the arrangement of atoms around a chiral center. Levorotatory and dextrorotatory refer to the rotation of light (either clockwise or counter clockwise), which can only be calculated experimentally using a polarimeter to create plane polarized light. "Rotates light clockwise" is simply another way to say dextrorotatory. Therefore, none of the answer choices are correct.
S configuration deals with the arrangement of atoms around a chiral center. Levorotatory and dextrorotatory refer to the rotation of light (either clockwise or counter clockwise), which can only be calculated experimentally using a polarimeter to create plane polarized light. "Rotates light clockwise" is simply another way to say dextrorotatory. Therefore, none of the answer choices are correct.
← Didn't Know|Knew It →

How many stereoisomers exist for the given compound?

How many stereoisomers exist for the given compound?
Tap to reveal answer
In this question, we're presented with the structure of a compound and we're asked to determine how many stereoisomers for this compound exists. It's important to remember that stereoisomers are compounds that have the same chemical formula and the same connectivity between its atoms, but what sets them apart is how their atoms are oriented in space.
When looking at the structure of the molecule in the question, we can see that there are two chiral carbons (carbons with four different substituents bound). A chiral carbon can have its substituents bound in two different ways, either R or S. Since each chiral carbon has two possible configurations of its atoms, the total numbers of possible stereoisomers is equal to  , where
, where  is equal to the number of chiral carbons. Thus, we can conclude that the number of stereoisomers is equal to
is equal to the number of chiral carbons. Thus, we can conclude that the number of stereoisomers is equal to  .
.
In this question, we're presented with the structure of a compound and we're asked to determine how many stereoisomers for this compound exists. It's important to remember that stereoisomers are compounds that have the same chemical formula and the same connectivity between its atoms, but what sets them apart is how their atoms are oriented in space.
When looking at the structure of the molecule in the question, we can see that there are two chiral carbons (carbons with four different substituents bound). A chiral carbon can have its substituents bound in two different ways, either R or S. Since each chiral carbon has two possible configurations of its atoms, the total numbers of possible stereoisomers is equal to 


← Didn't Know|Knew It →

How many different stereoisomer orientations are possible for the given molecule?

How many different stereoisomer orientations are possible for the given molecule?
Tap to reveal answer
There are 5 different chiral centers in the molecule as shown below:

In order for a carbon to be a chiral center, it must be bonded to 4 different groups. The total number of possible stereoisomers is equal to  , where n is the number of chiral centers. So in this case,
, where n is the number of chiral centers. So in this case, 
There are 5 different chiral centers in the molecule as shown below:

In order for a carbon to be a chiral center, it must be bonded to 4 different groups. The total number of possible stereoisomers is equal to 
← Didn't Know|Knew It →

How many chiral centers does the given molecule have?

How many chiral centers does the given molecule have?
Tap to reveal answer
There are 8 chiral centers which are marked below:

Carbon atoms need to be attached to 4 different groups to have a chiral center. Keep in mind that carbon atoms with a double bond can never be chiral. Looking at chiral center 1, the carbon is bonded to an alcohol group, a hydrogen atom, and two hydrocarbon groups. The hydrocarbon group clockwise is not identical to the hydrocarbon group counterclockwise. So this carbon would be considered bonded to 4 different groups making it chiral.
There are 8 chiral centers which are marked below:

Carbon atoms need to be attached to 4 different groups to have a chiral center. Keep in mind that carbon atoms with a double bond can never be chiral. Looking at chiral center 1, the carbon is bonded to an alcohol group, a hydrogen atom, and two hydrocarbon groups. The hydrocarbon group clockwise is not identical to the hydrocarbon group counterclockwise. So this carbon would be considered bonded to 4 different groups making it chiral.
← Didn't Know|Knew It →

In the given molecule, what are the orientations of the top and bottom carbons respectively?

In the given molecule, what are the orientations of the top and bottom carbons respectively?
Tap to reveal answer
The orientation of the chiral center is based on what the carbon is bonded to. The heaviest atom that the carbon is bonded is given higher priority.
Top: Bottom:
Bottom:
For the top carbon the oxygen is the heaviest, so it receives a 1, with the hydrogen as the least important group 4. The least priority group should be placed in the back, such as shown in the bottom example, before determining clockwise or counterclockwise orientation. For the bottom section, going from most important to least important groups, while ignoring the least important group, you get clock wise or R orientation.
Interpreting the top carbon is different because the least important group is not in the back. Reading from high to low priority, while the hydrogen is in the front, gives a S configuration (ignore the 4th priority group when rotating). Since the hydrogen group is opposite from where it should be, the orientation is opposite as well.
The orientation of the chiral center is based on what the carbon is bonded to. The heaviest atom that the carbon is bonded is given higher priority.
Top: Bottom:
Bottom:
For the top carbon the oxygen is the heaviest, so it receives a 1, with the hydrogen as the least important group 4. The least priority group should be placed in the back, such as shown in the bottom example, before determining clockwise or counterclockwise orientation. For the bottom section, going from most important to least important groups, while ignoring the least important group, you get clock wise or R orientation.
Interpreting the top carbon is different because the least important group is not in the back. Reading from high to low priority, while the hydrogen is in the front, gives a S configuration (ignore the 4th priority group when rotating). Since the hydrogen group is opposite from where it should be, the orientation is opposite as well.
← Didn't Know|Knew It →

What is the orientation of the given molecule?
What is the orientation of the given molecule?
Tap to reveal answer
Label the priority of bonded groups first.

Moving from first to second to third, which ignoring the 4th important group, gives a counterclockwise direction, or S.
Label the priority of bonded groups first.

Moving from first to second to third, which ignoring the 4th important group, gives a counterclockwise direction, or S.
← Didn't Know|Knew It →
Isomers that are nonsuperimposeable mirror images of each other are called .
Isomers that are nonsuperimposeable mirror images of each other are called .
Tap to reveal answer
Enantiomers are chiral isomers of the same molecule that are mirror images of one another. Because of this characteristic, enantiomers cannot be placed on top of one another (superimposed) and yield the same molecule.
Enantiomers are chiral isomers of the same molecule that are mirror images of one another. Because of this characteristic, enantiomers cannot be placed on top of one another (superimposed) and yield the same molecule.
← Didn't Know|Knew It →
What is the IUPAC name for the given organic molecule?

What is the IUPAC name for the given organic molecule?

Tap to reveal answer
The absolute configuration around carbons 2 and 4 is R and S, respectively. The molecule is a hexanol, and the numbering starts with the carbon closest to the hydroxyl group.
The absolute configuration around carbons 2 and 4 is R and S, respectively. The molecule is a hexanol, and the numbering starts with the carbon closest to the hydroxyl group.
← Didn't Know|Knew It →
Which of the following structures is equivalent to the enantiomer of the molecule whose Fischer Projection is shown below?


Which of the following structures is equivalent to the enantiomer of the molecule whose Fischer Projection is shown below?


Tap to reveal answer
The compound shown is SS, so we need to look for the compound that is RR.
The compound shown is SS, so we need to look for the compound that is RR.
← Didn't Know|Knew It →

How many stereocenters does the molecular framework of cholic acid (shown) have?

How many stereocenters does the molecular framework of cholic acid (shown) have?
Tap to reveal answer

There are 11 stereocenters, because here there are 11 asymmetric carbons and no E/Z isomerisms, nor planes of symmetry.

There are 11 stereocenters, because here there are 11 asymmetric carbons and no E/Z isomerisms, nor planes of symmetry.
← Didn't Know|Knew It →

How many possible stereoisomers does the molecular framework of cholic acid (shown) have?

How many possible stereoisomers does the molecular framework of cholic acid (shown) have?
Tap to reveal answer

There are 11 stereocenters, because here there are 11 asymmetric carbons and no E/Z isomerisms, nor planes of symmetry. For compounds with no meso isomers or E/Z isomerisms, the possible number of stereoisomers is  where
where  is the number of stereocenters.
is the number of stereocenters.

There are 11 stereocenters, because here there are 11 asymmetric carbons and no E/Z isomerisms, nor planes of symmetry. For compounds with no meso isomers or E/Z isomerisms, the possible number of stereoisomers is 

← Didn't Know|Knew It →

How many stereocenters does this steroid derivative have?

How many stereocenters does this steroid derivative have?
Tap to reveal answer
There are 11 asymmetric carbons and one E double bond, so there are 13 stereocenters in total. For each E/Z isomerism, there are 2 stereocenters.

There are 11 asymmetric carbons and one E double bond, so there are 13 stereocenters in total. For each E/Z isomerism, there are 2 stereocenters.

← Didn't Know|Knew It →

How many possible stereoisomers does the following steroid derivative have?

How many possible stereoisomers does the following steroid derivative have?
Tap to reveal answer
Here there are 11 asymmetric carbons and one E/Z isomerism.
So, the number of possible stereocenters from asymmetric carbons is  , and the number of possible stereoisomers from E/Z isomerisms is
, and the number of possible stereoisomers from E/Z isomerisms is  .
.
Thus the number of stereoisomers from asymmetric carbons is doubled, and 
 possible stereoisomers
possible stereoisomers

Here there are 11 asymmetric carbons and one E/Z isomerism.
So, the number of possible stereocenters from asymmetric carbons is 

Thus the number of stereoisomers from asymmetric carbons is doubled, and 


← Didn't Know|Knew It →

Assign absolute configuration to the tetrahedral asymmetric stereocenter (TAS) circled in red.

Assign absolute configuration to the tetrahedral asymmetric stereocenter (TAS) circled in red.
Tap to reveal answer
Aside from the hydrogen that extends "back into the page", priority 1 goes to the top right group (from the perspective of the TAS) because of the large chlorine atom. Priority 2 goes to the top left group because of the carbon atom attached to another carbon atom. Priority 3 goes to the methyl group because it is a carbon atom attached to hydrogen atoms, which have the lowest priority. When the hydrogen atom attached to the TAS is viewed as going back into the page, the circle created by going from priority 1 to priority 3 is counterclockwise, so we assign this TAS to be S. Cis and trans are irrelevant to TAS.
Aside from the hydrogen that extends "back into the page", priority 1 goes to the top right group (from the perspective of the TAS) because of the large chlorine atom. Priority 2 goes to the top left group because of the carbon atom attached to another carbon atom. Priority 3 goes to the methyl group because it is a carbon atom attached to hydrogen atoms, which have the lowest priority. When the hydrogen atom attached to the TAS is viewed as going back into the page, the circle created by going from priority 1 to priority 3 is counterclockwise, so we assign this TAS to be S. Cis and trans are irrelevant to TAS.
← Didn't Know|Knew It →

Assign absolute configuration to the tetrahedral asymmetric center (TAS) circled in blue.

Assign absolute configuration to the tetrahedral asymmetric center (TAS) circled in blue.
Tap to reveal answer
Aside from the hydrogen that extends "forward from the page", priority 1 goes to the top left group (from the perspective of the TAS) because of the chlorine atom. Priority 2 goes to the bottom left group because of the carbon atom attached to another two carbon atoms. Priority 3 goes to the bottom right group, which has a carbon attached to only one other carbon at the point of difference with the group that has priority 2. When the hydrogen atom attached to the TAS is viewed as going back into the page, the circle created by going from priority 1 to priority 3 is clockwise, so we assign this TAS to be R. Cis and trans are irrelevant to TAS.
Aside from the hydrogen that extends "forward from the page", priority 1 goes to the top left group (from the perspective of the TAS) because of the chlorine atom. Priority 2 goes to the bottom left group because of the carbon atom attached to another two carbon atoms. Priority 3 goes to the bottom right group, which has a carbon attached to only one other carbon at the point of difference with the group that has priority 2. When the hydrogen atom attached to the TAS is viewed as going back into the page, the circle created by going from priority 1 to priority 3 is clockwise, so we assign this TAS to be R. Cis and trans are irrelevant to TAS.
← Didn't Know|Knew It →
What is the Fischer projection of the following molecule?


What is the Fischer projection of the following molecule?


Tap to reveal answer
The absolute configuration of the given molecule is  (amino group is R, methyl group is S). Putting the methyl group on the top and the alcohol on the bottom, this puts the amino group on the left, and the methyl group on the right. Recall that in the Fischer projection, the vertical bonds are dashed and are going into the plane of the screen/paper, while the horizontal bonds are wedges and are coming out of the plane of the screen/paper. One way to remember this is to think of Fischer projections as a skeleton (dashed vertical) wearing a bowtie (wedged horizontal).
(amino group is R, methyl group is S). Putting the methyl group on the top and the alcohol on the bottom, this puts the amino group on the left, and the methyl group on the right. Recall that in the Fischer projection, the vertical bonds are dashed and are going into the plane of the screen/paper, while the horizontal bonds are wedges and are coming out of the plane of the screen/paper. One way to remember this is to think of Fischer projections as a skeleton (dashed vertical) wearing a bowtie (wedged horizontal).
The absolute configuration of the given molecule is 
← Didn't Know|Knew It →