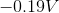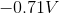Oxidation-Reduction
Help Questions
MCAT Physical › Oxidation-Reduction
The standard reduction potentials for iron and nickel are as follows:


If a galvanic cell is made using these two metals, what is the standard voltage for the cell?
Explanation
A galvanic cell is spontaneous, meaning that there will be a positive cell voltage. In order for this to take place, the more negative reduction potential must be flipped so that the metal is oxidized.


Adding these values together gives a standard cell voltage of 
The standard reduction potentials for iron and nickel are as follows:


If a galvanic cell is made using these two metals, what is the standard voltage for the cell?
Explanation
A galvanic cell is spontaneous, meaning that there will be a positive cell voltage. In order for this to take place, the more negative reduction potential must be flipped so that the metal is oxidized.


Adding these values together gives a standard cell voltage of 
What is the oxidation number of chromium in calcium dichromate (CaCr2O7)?
+6
+2
0
+7
-2
Explanation
Since the calcium ion has a charge of +2, the dichromate anion must have a charge of -2. Oxygen always has an oxidation number of -2, so the seven oxygens in the molecule have a total number of -14. That leaves a charge of +12 that the two chromiums must balance out (-2 - (-14) = 12). Each chromium must therefore have an oxidation number of +6.
In the above unbalanced redox reaction, which best describes the oxidation/reduction roles of iron and oxygen?
Iron is oxidized, oxygen is reduced
Iron is reduced, oxygen is oxidized
Iron is oxidized, oxygen is neither oxidized nor reduced
Iron is reduced, oxygen is neither oxidized nor reduced
Iron is reduced, oxygen is reduced
Explanation
There are a couple options to solve this problem. First, we could simply remember that anything which combines with oxygen in a redox reaction is oxidized, and the associated oxygen is reduced.
Another option is to think about the oxidation numbers of each element (an element whose oxidation number increases is oxidized, and one whose oxidation number decreases is reduced). If we choose this method, recall that free elements (Fe and O2 in this case) have oxidation number 0. Also, oxygen in compounds has oxidation number -2. Oxygen's oxidation number decreases from 0 to -2, meaning that oxygen is reduced. Since oxidation and reduction occur at the same time in different elements of the redox reaction, iron's oxidation number must increase, so iron is oxidized.
Which element in the given compounds has the highest oxidation number?
Sulfur in
Carbon in
Aluminum in
Sulfur in
Oxygen in
Explanation
We can calculate the oxidation number in each answer to find the greatest value.
In 


In 


In 


In 




What is the correct oxidation number of zinc in the compound 
+2
-2
+1
-1
0
Explanation
The sum of oxidation numbers of each element in this compound must add to the total charge of -2. Hydrogen always has an oxidation number of +1, and oxygen always has an oxidation number of -2.
So, in total for oxygen we have (4) * (-2) = -8, and for hydrogen we have (4) * (+1) = +4.
Combining the oxygen and hydrogen, (+4) + (-8) = -4, so we need an additional +2 to achieve the total charge of -2. Zn must provide this balancing charge, and have an oxidation number of +2.
What is the oxidation number of chromium in calcium dichromate (CaCr2O7)?
+6
+2
0
+7
-2
Explanation
Since the calcium ion has a charge of +2, the dichromate anion must have a charge of -2. Oxygen always has an oxidation number of -2, so the seven oxygens in the molecule have a total number of -14. That leaves a charge of +12 that the two chromiums must balance out (-2 - (-14) = 12). Each chromium must therefore have an oxidation number of +6.
In the above unbalanced redox reaction, which best describes the oxidation/reduction roles of iron and oxygen?
Iron is oxidized, oxygen is reduced
Iron is reduced, oxygen is oxidized
Iron is oxidized, oxygen is neither oxidized nor reduced
Iron is reduced, oxygen is neither oxidized nor reduced
Iron is reduced, oxygen is reduced
Explanation
There are a couple options to solve this problem. First, we could simply remember that anything which combines with oxygen in a redox reaction is oxidized, and the associated oxygen is reduced.
Another option is to think about the oxidation numbers of each element (an element whose oxidation number increases is oxidized, and one whose oxidation number decreases is reduced). If we choose this method, recall that free elements (Fe and O2 in this case) have oxidation number 0. Also, oxygen in compounds has oxidation number -2. Oxygen's oxidation number decreases from 0 to -2, meaning that oxygen is reduced. Since oxidation and reduction occur at the same time in different elements of the redox reaction, iron's oxidation number must increase, so iron is oxidized.
Which element in the given compounds has the highest oxidation number?
Sulfur in
Carbon in
Aluminum in
Sulfur in
Oxygen in
Explanation
We can calculate the oxidation number in each answer to find the greatest value.
In 


In 


In 


In 




What is the correct oxidation number of zinc in the compound 
+2
-2
+1
-1
0
Explanation
The sum of oxidation numbers of each element in this compound must add to the total charge of -2. Hydrogen always has an oxidation number of +1, and oxygen always has an oxidation number of -2.
So, in total for oxygen we have (4) * (-2) = -8, and for hydrogen we have (4) * (+1) = +4.
Combining the oxygen and hydrogen, (+4) + (-8) = -4, so we need an additional +2 to achieve the total charge of -2. Zn must provide this balancing charge, and have an oxidation number of +2.