Thermodynamic Systems and Calorimetry
Help Questions
MCAT Physical › Thermodynamic Systems and Calorimetry
The combustion of liquid hexane in air at 298K gives gaseous carbon dioxide and liquid water, as shown in this reaction.




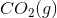


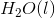

Calculate the 
Explanation
To calculate the 

Now we can plug in the given values and solve for the enthalpy of reaction.
Use the following values for water as needed.
If burning wood releases 



Explanation
There are two processes requiring added heat in this problem:
1. Raising the temperature of the liquid water from 


2. Boiling the water at a constant temperature of 

To use either of these equation, we need to find the mass of the water using the relation between mass, density, and volume.
Use this mass with the given specific heat and temperatures to find the heat for part 1 of the process.
Then, use the mass with the given heat of vaporization to find the energy needed to convert the water to water vapor.
Sum the energies for step 1 and step 2.
This is the total amount of energy needed from the burning wood. Use stoichiometry to find the grams of wood needed to produce this amount of energy.
A scientist prepares an experiment to demonstrate the second law of thermodynamics for a chemistry class. In order to conduct the experiment, the scientist brings the class outside in January and gathers a cup of water and a portable stove.
The temperature outside is –10 degrees Celsius. The scientist asks the students to consider the following when answering his questions:
Gibbs Free Energy Formula:
ΔG = ΔH – TΔS
Liquid-Solid Water Phase Change Reaction:
H2O(l) ⇌ H2O(s) + X
The scientist prepares two scenarios.
Scenario 1:
The scientist buries the cup of water outside in the snow, returns to the classroom with his class for one hour, and the class then checks on the cup. They find that the water has frozen in the cup.
Scenario 2:
The scientist then places the frozen cup of water on the stove and starts the gas. The class finds that the water melts quickly.
After the water melts, the scientist asks the students to consider two hypothetical scenarios as a thought experiment.
Scenario 3:
Once the liquid water at the end of scenario 2 melts completely, the scientist turns off the gas and monitors what happens to the water. Despite being in the cold air, the water never freezes.
Scenario 4:
The scientist takes the frozen water from the end of scenario 1, puts it on the active stove, and the water remains frozen.
In this situation described in the passage, which of the following is true?
The cup of water is the system, and the snow, stove, and remainder of the universe are the surroundings.
The cup of water is the system, and the stove is the surroundings.
The cup of water is the system, and the snow is the surroundings.
The cup of water, snow, and stove comprise the system; the universe is the surroundings
The system is all of the universe, except the stove used to input energy.
Explanation
The Gibbs Free Energy equation makes use of clearly delineated systems and surroundings. In this example, the water is freezing or melting depending on conditions. This is accompanied by thermal exchanges with other players, such as the snow and stove. Thus, the water is the system, and everything else (technically, everything else in the universe) comprises the surroundings.
The combustion of propane is given by the following formula.
If the heats of formation for CO, CO2, H2O, and C3H8 are -110.5 kJ/mol, -393.5 kJ/mol, -241.8 kJ/mol, and -103.85 kJ/mol, respectively, what is the heat of reaction for the combustion of propane?
–1477.9kJ
1477.9kJ
–641.95kJ
641.95kJ
Explanation
Given the fact that combustion reactions are exothermic, you should expect the heat of reaction to be negative (ruling out two answer choices). The heat of reaction is equal to the heat of formation of the products minus the heat of formation of the reactants. Be sure to refer to the balanced equation for the correct number of moles for each compound.
Oxygen is not included, as it is in elemental form and therefore has a heat of formation equal to zero.
Given the enthalpies of formation, what is the enthalpy of combustion of octane in the reaction:
Explanation
The equation for enthalpy of reaction is:
Given our chemical reaction and the enthalpies of formation, we can find the enthalpy of reaction.
First, find the total enthalpy for the products.
Then, find the total enthalpy for the reactants.
Since the oxygen is elemental, its heat of formation is zero.
Return to the original equation to calculate the final enthalpy of reaction.

In the above reaction, how much heat will be released if 74.0g of sulfur reacts with excess oxygen? Round to the nearest 10kJ.
Explanation
Basically, this is a unit conversion problem. Starting with grams of sulfur, convert to moles of sulfur, and finally to kJ of heat released. Note that the given enthalpy of reaction, 
Note that a negative enthalpy of reaction means the process is exothermic and releases heat, while a positive enthalpy of reaction would mean the process is endothermic and absorbs heat.
A 35g piece of aluminum at a temperature of 373K is placed into 150g of water at a temperature of 298K. The aluminum and water eventually become the same temperature. No heat is released to the surroundings.
Water has a specific heat capacity of 

What is the final temperature of the water and aluminum in the container?
301.6K
324.5K
315.5K
308.9K
Explanation
In this problem, we need to track the transfer of heat from the aluminum to the water. Since the heat acquired by the water is equal to the heat given off by the aluminum, we can set their equations equal to each other; however, in order to avoid a negative number, aluminum's change in temperature will be set as the initial temperature minus the final temperature. This results in the equation below.

How much energy must a stove transfer to completely transform 

Explanation
To turn the water into steam, the stove must first raise the temperature of the water, and then provide energy to change the phase. This requires two distinct steps.
Find the energy to raise the temperature of the water using the equation 
Now, we need to find the energy needed to convert the water to a gas. We will use the equation 
Finally, we add the energy for the two steps to find the total energy required.
A 50g sample of an unknown substance is heated to 100oC in a tub of boiling water. It is then quickly removed and placed into an insulated jar holding 200mL of water, initially at 20oC. The final equilbrium temperature of the system is 30oC. Approximately, what is the specific heat of this unknown substance?
Specific heat of water is 4.187J/goC.
Density of water is 1g/mL.
Explanation
Since the system is isolated, the amount of heat transferred away from the unknown substance must equal the heat transferred to the water. To calculate these heats, use
For the water, 
For the unknown substance,
Set these values equal to get cx = 2.39 J/goC, approximately 2.4 J/goC.
A 200g sample of gold is subjected to 1.2kJ of heat.
The specific heat capacity for gold is 
What is the change in temperature as a result of the heating?
Explanation
When a specific heat capacity is given, we typically use the equation 



















































































