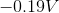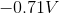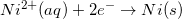Reduction Potentials
Help Questions
MCAT Physical › Reduction Potentials
The standard reduction potentials for iron and nickel are as follows:


If a galvanic cell is made using these two metals, what is the standard voltage for the cell?
Explanation
A galvanic cell is spontaneous, meaning that there will be a positive cell voltage. In order for this to take place, the more negative reduction potential must be flipped so that the metal is oxidized.


Adding these values together gives a standard cell voltage of 
Consider the half reaction below.
Which of the following statements is true about the oxidation of nickel?
The oxidation of nickel has a potential of 0.23V
It is not possible to oxidize nickel
The oxidation of nickel has a potential of 0V
The nickel will gain electrons when oxidized
None of these are true
Explanation
Reduction potentials are typically provided in table form. Since the oxidation potential of an element is the negative of the reduction potential, it can be determined by viewing a reduction table.
Since the reduction potential for nickel is given as –0.23V, we can conclude that the oxidation potential for nickel is 0.23V.