Thermochemistry and Energetics
Help Questions
MCAT Physical › Thermochemistry and Energetics
Boiling point is the temperature a liquid needs to achieve in order to begin its transformation into a gaseous state. Campers and hikers who prepare food during their trips have to account for differences in atmospheric pressure as they ascend in elevation. During the ascent, the decrease in atmospheric pressure changes the temperature at which water boils.
Further complicating the matter is the observation that addition of a solute to a pure liquid also changes the boiling point. Raoult’s Law can be used to understand the changes in boiling point if a non-volatile solute is present, as expressed here.
In this law, 


A scientist is studying solution chemistry to better understand vapor pressure. He finds that, for one solution he creates, the beaker is cool to the touch after the solute is fully dissolved. Which of the following is true of this solution? (Note: The beaker, solute, and solvent are the system, the remainder of the universe is the surroundings)
It forms spontaneously only at high temperatures
It forms spontaneously only at low temperatures
It always forms spontaneously
It never forms spontaneously
It forms spontaneously only if dissolution decreases entropy of the system
Explanation
The act of dissolving a solute in a solvent is a local increase in entropy, converting a single molecule to multiple ions. The absorption of heat from the surroundings (cool beaker) indicates that this is an endothermic dissolution. We can look at the equation for Gibbs free energy to evaluate the possible answers.
In order to be spontaneous, the reaction must have a negative Gibbs free energy. To accomplish this, a reaction may have a negative enthalpy (exothermic) and positive entropy, however we know that our reaction has a positive enthalpy (endothermic) and positive entropy. A reaction will be spontaneous if it has a positive 

A scientist prepares an experiment to demonstrate the second law of thermodynamics for a chemistry class. In order to conduct the experiment, the scientist brings the class outside in January and gathers a cup of water and a portable stove.
The temperature outside is –10 degrees Celsius. The scientist asks the students to consider the following when answering his questions:
Gibbs Free Energy Formula:
ΔG = ΔH – TΔS
Liquid-Solid Water Phase Change Reaction:
H2O(l) ⇌ H2O(s) + X
The scientist prepares two scenarios.
Scenario 1:
The scientist buries the cup of water outside in the snow, returns to the classroom with his class for one hour, and the class then checks on the cup. They find that the water has frozen in the cup.
Scenario 2:
The scientist then places the frozen cup of water on the stove and starts the gas. The class finds that the water melts quickly.
After the water melts, the scientist asks the students to consider two hypothetical scenarios as a thought experiment.
Scenario 3:
Once the liquid water at the end of scenario 2 melts completely, the scientist turns off the gas and monitors what happens to the water. Despite being in the cold air, the water never freezes.
Scenario 4:
The scientist takes the frozen water from the end of scenario 1, puts it on the active stove, and the water remains frozen.
The same scientist in the passage measures the variables of another reaction in the lab. He knows that this reaction is spontaneous under standard conditions, with a standard free energy change of –43 kJ/mol. Using laboratory-calculated variables, he determines that the Gibbs Free Energy has a value of 0 kJ/mol. What can we say about this reaction?
The reaction had reached equilibrium when the scientist made his measurements.
It indicates that the reaction will produce 43 kJ of energy for every mole that is allowed to react spontaneously under current conditions.
43 kJ of energy must be put into the system to have the reaction proceed spontaneously.
The scientist made an error, all spontaneous reactions have a negative Gibbs Free Energy value.
The reaction will always produce 43 kJ of heat when it reacts to completion.
Explanation
A reaction with a zero free energy change is at equilibrium. At standard conditions, not at equilibrium, this reaction would have a Gibbs free energy change of –43 kJ/mol, would be spontaneous, and would be able to produce 43 kJ of useful work for every mole that reacts.
A scientist prepares an experiment to demonstrate the second law of thermodynamics for a chemistry class. In order to conduct the experiment, the scientist brings the class outside in January and gathers a cup of water and a portable stove.
The temperature outside is –10 degrees Celsius. The scientist asks the students to consider the following when answering his questions:
Gibbs Free Energy Formula:
ΔG = ΔH – TΔS
Liquid-Solid Water Phase Change Reaction:
H2O(l) ⇌ H2O(s) + X
The scientist prepares two scenarios.
Scenario 1:
The scientist buries the cup of water outside in the snow, returns to the classroom with his class for one hour, and the class then checks on the cup. They find that the water has frozen in the cup.
Scenario 2:
The scientist then places the frozen cup of water on the stove and starts the gas. The class finds that the water melts quickly.
After the water melts, the scientist asks the students to consider two hypothetical scenarios as a thought experiment.
Scenario 3:
Once the liquid water at the end of scenario 2 melts completely, the scientist turns off the gas and monitors what happens to the water. Despite being in the cold air, the water never freezes.
Scenario 4:
The scientist takes the frozen water from the end of scenario 1, puts it on the active stove, and the water remains frozen.
The same scientist in the passage measures the variables of another reaction in the lab. He knows that this reaction is spontaneous under standard conditions, with a standard free energy change of –43 kJ/mol. Using laboratory-calculated variables, he determines that the Gibbs Free Energy has a value of 0 kJ/mol. What can we say about this reaction?
The reaction had reached equilibrium when the scientist made his measurements.
It indicates that the reaction will produce 43 kJ of energy for every mole that is allowed to react spontaneously under current conditions.
43 kJ of energy must be put into the system to have the reaction proceed spontaneously.
The scientist made an error, all spontaneous reactions have a negative Gibbs Free Energy value.
The reaction will always produce 43 kJ of heat when it reacts to completion.
Explanation
A reaction with a zero free energy change is at equilibrium. At standard conditions, not at equilibrium, this reaction would have a Gibbs free energy change of –43 kJ/mol, would be spontaneous, and would be able to produce 43 kJ of useful work for every mole that reacts.
Boiling point is the temperature a liquid needs to achieve in order to begin its transformation into a gaseous state. Campers and hikers who prepare food during their trips have to account for differences in atmospheric pressure as they ascend in elevation. During the ascent, the decrease in atmospheric pressure changes the temperature at which water boils.
Further complicating the matter is the observation that addition of a solute to a pure liquid also changes the boiling point. Raoult’s Law can be used to understand the changes in boiling point if a non-volatile solute is present, as expressed here.
In this law, 


A scientist is studying solution chemistry to better understand vapor pressure. He finds that, for one solution he creates, the beaker is cool to the touch after the solute is fully dissolved. Which of the following is true of this solution? (Note: The beaker, solute, and solvent are the system, the remainder of the universe is the surroundings)
It forms spontaneously only at high temperatures
It forms spontaneously only at low temperatures
It always forms spontaneously
It never forms spontaneously
It forms spontaneously only if dissolution decreases entropy of the system
Explanation
The act of dissolving a solute in a solvent is a local increase in entropy, converting a single molecule to multiple ions. The absorption of heat from the surroundings (cool beaker) indicates that this is an endothermic dissolution. We can look at the equation for Gibbs free energy to evaluate the possible answers.
In order to be spontaneous, the reaction must have a negative Gibbs free energy. To accomplish this, a reaction may have a negative enthalpy (exothermic) and positive entropy, however we know that our reaction has a positive enthalpy (endothermic) and positive entropy. A reaction will be spontaneous if it has a positive 

A student is performing a reaction with unknown compounds in his chemistry lab. The only information the student knows about the reaction is that it is endothermic and reversible. Using this knowledge alone, how can the student increase the yield of his product?
Increase the temperature
Decrease the temperature
Add product to the reaction
Remove reactant from the reaction
Explanation
To answer this question, we need to have a solid understanding of Le Chatelier's principle.
In an endothermic reaction, heat is needed to facilitate the reaction. To increase the products, we want to shift the reaction to the right.
We should already know that adding product or removing reactant shifts the equilibrium to the left, and yields more starting material rather than product. In an endothermic reaction, we can consider heat as a reactant; thus, adding heat (increasing temperature) would allow us to shift the reaction to the right.
Decreasing the temperature, removing reactant, or adding product would all increase the yield of the starting materials.
For any given chemical reaction, one can draw an energy diagram. Energy diagrams depict the energy levels of the different steps in a reaction, while also indicating the net change in energy and giving clues to relative reaction rate.
Below, a reaction diagram is shown for a reaction that a scientist is studying in a lab. A student began the reaction the evening before, but the scientist is unsure as to the type of the reaction. He cannot find the student’s notes, except for the reaction diagram below.

Growing frustrated by his inability to decipher what chemical reaction the student had started, the scientist decides to measure the reaction vessel's temperature. Based only on the above reaction diagram, what is he most likely to find if the reaction is ongoing?
(Assume that the reaction vessel is defined as the system, and that the entropy of the system decreases)
A warm container from an exothermic reaction
A cold container from an exothermic reaction
A warm container from an endothermic reaction
A cold container from an endothermic reaction
The products of this reaction have an equal energy level to the reactants
Explanation
Point 5 is the energy level of the products of the reaction, while point 1 is the energy level of the reactants. Point 5 is lower than is point 1, indicating that the products of this reaction contain lower overall energy than do the reactants. This energy must be released in some form, likely as heat, characteristic of an exothermic reaction.
The question further specifies that there is a local decrease in entropy of the system, thus, the only way that entropy of the universe can increase is to release heat and increase the entropy of the surroundings.
The combustion of liquid hexane in air at 298K gives gaseous carbon dioxide and liquid water, as shown in this reaction.




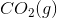


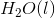

Calculate the 
Explanation
To calculate the 

Now we can plug in the given values and solve for the enthalpy of reaction.
The combustion of liquid hexane in air at 298K gives gaseous carbon dioxide and liquid water, as shown in this reaction.




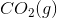


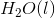

Calculate the 
Explanation
To calculate the 

Now we can plug in the given values and solve for the enthalpy of reaction.
For any given chemical reaction, one can draw an energy diagram. Energy diagrams depict the energy levels of the different steps in a reaction, while also indicating the net change in energy and giving clues to relative reaction rate.
Below, a reaction diagram is shown for a reaction that a scientist is studying in a lab. A student began the reaction the evening before, but the scientist is unsure as to the type of the reaction. He cannot find the student’s notes, except for the reaction diagram below.

Growing frustrated by his inability to decipher what chemical reaction the student had started, the scientist decides to measure the reaction vessel's temperature. Based only on the above reaction diagram, what is he most likely to find if the reaction is ongoing?
(Assume that the reaction vessel is defined as the system, and that the entropy of the system decreases)
A warm container from an exothermic reaction
A cold container from an exothermic reaction
A warm container from an endothermic reaction
A cold container from an endothermic reaction
The products of this reaction have an equal energy level to the reactants
Explanation
Point 5 is the energy level of the products of the reaction, while point 1 is the energy level of the reactants. Point 5 is lower than is point 1, indicating that the products of this reaction contain lower overall energy than do the reactants. This energy must be released in some form, likely as heat, characteristic of an exothermic reaction.
The question further specifies that there is a local decrease in entropy of the system, thus, the only way that entropy of the universe can increase is to release heat and increase the entropy of the surroundings.
A student is performing a reaction with unknown compounds in his chemistry lab. The only information the student knows about the reaction is that it is endothermic and reversible. Using this knowledge alone, how can the student increase the yield of his product?
Increase the temperature
Decrease the temperature
Add product to the reaction
Remove reactant from the reaction
Explanation
To answer this question, we need to have a solid understanding of Le Chatelier's principle.
In an endothermic reaction, heat is needed to facilitate the reaction. To increase the products, we want to shift the reaction to the right.
We should already know that adding product or removing reactant shifts the equilibrium to the left, and yields more starting material rather than product. In an endothermic reaction, we can consider heat as a reactant; thus, adding heat (increasing temperature) would allow us to shift the reaction to the right.
Decreasing the temperature, removing reactant, or adding product would all increase the yield of the starting materials.













