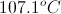Boiling Point
Help Questions
MCAT Physical › Boiling Point
Colligative properties are properties of compounds that are altered by the amount of substance present. There are four main colligative properties: boiling point, freezing point, vapor pressure, and osmotic pressure. The change in each of these properties can be calculated using the amount of molecules/ions present in solution and the concentration or partial pressure of the compound. The boiling point is defined as the temperature at which the vapor pressure equals the atmospheric pressure. The freezing point is the temperature at which a liquid is converted to a solid. Vapor pressure is the pressure produced by the vapor above a solution. Osmotic pressure is the pressure required to prevent flow of water into a solution (across a membrane).
Which of the following is true regarding the boiling point of a sodium chloride solution and calcium chloride solution?
The relative boiling points cannot be determined because the concentration is not given
The boiling point of calcium chloride solution will be higher
The boiling point of sodium chloride solution will be higher
The boiling point of both solutions will be equal
Explanation
Recall that the boiling point of a solution depends on the number of ions in the solution and the concentration of the solution. Increase in both of these factors will elevate the boiling point to higher temperatures. The equation describing this is given below.
where 





Which of the following compounds will create the greatest increase in boiling point when added to an aqueous solution?
Explanation
Colligative properties are dependent only on the number of particles in a solution, and not their identity. Some examples of colligative properties are vapor pressure, boiling point, freezing point, and osmotic pressure.
There is a direct relationship between the boiling point elevation and the number of particles present in a solution. The more particles that are present in solution, the higher the boiling point elevation.
We are looking for the compound that will create the greatest number of ions when dissolved in solution.
Sodium chloride and magnesium sulfate will produce two ions per mole.
Magnesium chloride and barium chloride will produce three ions per mole.
Calcium hydroxide will also produce three ions per mole, but we are given two moles instead of one.
Calcium hydroxide will produce the greatest number of ions, thus creating the greatest increase in boiling point elevation.
Boiling point is the temperature a liquid needs to achieve in order to begin its transformation into a gaseous state. Campers and hikers who prepare food during their trips have to account for differences in atmospheric pressure as they ascend in elevation. During the ascent, the decrease in atmospheric pressure changes the temperature at which water boils.
Further complicating the matter is the observation that addition of a solute to a pure liquid also changes the boiling point. Raoult’s Law can be used to understand the changes in boiling point if a non-volatile solute is present, as expressed here.
In this law, 


The change in boiling point with addition of a solute is a colligative property of a solution. Which of the following are also examples of colligative properties?
I. Vapor pressure reduction
II. Color emission with dissolution of a solute
III. Osmotic pressure
I and III, only
I, II, and III
III, only
II and III, only
I and II, only
Explanation
Colligative properties are defined as properties that depend entirely upon the ratio of the number of solute particles to the number of solvent particles. Only osmotic pressure and vapor pressure depression are examples of such phenomena. While color emission is a property of a solution, it depends on the chemical species involved, and not the number of particles.
The values for normal boiling and freezing points, along with 




Explanation
We first need to find the boiling point elevation with the equation:
Ammonium phosphate has an van't Hoff value of four; each molecule dissociates into four ions in solution. To calculate the molality, we need to find moles of solute per kilogram of solution.
Next, use the molality, van't Hoff factor, and boiling point elevation constant to solve for the increase in boiling point.
Add this increase to the boiling point of pure water to find the boiling point of the solution.
Two moles of sodium chloride (NaCl) are added to 1kg of a mystery solvent. The addition of the NaCl caused an increase of 6K to the solvent's boiling point.
Based on this information, what is the boiling constant for the solvent?
Explanation
In order to solve this problem, we can use the boiling point elevation equation: 
We know the temperature change, we can compute molality from the given information, and we know the van't Hoff factor (expected to be 2 in this scenario due to NaCl becoming 2 ions in solution). We can calculate the boiling point constant for the solvent.
Which of the following aqueous solutions will have the highest boiling point?
2m MgCl2
1m MgCl2
2m NaCl
1m NaF
Explanation
In order to answer this problem, consider the equation for boiling point elevation: 


Each of the following solutions is added to equal amounts of water. Which solution will result in the greatest amount of boiling point elevation?
Explanation
Boiling point elevation is a colligative property, meaning that it depends on the relative number of solute particles in solution. The answer choice with the largest number of moles of particles will show the greatest boiling point elevation. The equation for boiling point elevation is:
Molality is equal to moles of solute per kilogram of solvent, meaning that it will be proportional to the moles of solute added. Each solute is added to equal amounts of water, allowing us to keep this value constant. Similarly, 
Using this proportion, we can find the solute that will most impact the boiling point of water.
Since sodium chloride results in the greatest moles of ions in solution, it will yield the greatest boiling point elevation.
Which solution will have a higher boiling point?
Solution 1: 

Solution 2: 

Solution 1
Solution 2
Solution 1 and 2 will have the same boiling point
The answer cannot be determined from the information given
Explanation
Adding solute to water will result in boiling point elevation due to the presence of more molecules. Change in temperature is given by the relation 



Since 




The values for normal boiling and freezing points, along with 

A 
Magnesium phosphide in acetic acid
Sodium chloride in benzene
Sodium chloride in acetic acid
Magnesium phosphide in benzene
Explanation
Boiling point elevation depends on three variables: the boiling point elevation constant of the solvent, the van't Hoff factor of the solute, and the molality of the solution. In this question, molality is held constant.
First, calculate the van't Hoff for each compound.
Ultimately, we are looking for the greatest product of the boiling point elevation constant and van't Hoff factor (since molality is constant).
Magnesium phosphide has the greater van't Hoff factor and acetic acid has the greater boiling point elevation constant. A solution of magnesium phosphide in acetic acid will thus have the greatest boiling point elevation.
Suppose two containers each contain the same amount of solvent. 2m NaCl solution is added to the first container, and a mystery solution is added to the second container. Upon heating the flasks, it is determined that the second container has a higher boiling point than the first container. Assume the solutions are ideal.
Based on the above information, which of the following compounds could have been added to container 2?
2m CaF2
1m MgCl2
3m C6H12O6
1m NaCl
Explanation
Based on the equation 

The sodium choride added to container 1 has a molality of 2, as well as a van't Hoff factor of 2. As a result, we are looking for a compound that has a larger combination of these two factors, which would cause a higher boiling point. 2m CaF2 has a molality of 2 and a van't Hoff factor of 3. Since this combination of factors in container 2 would be higher than the combination in container 1, we can conclude that this was the mystery compound added to the container with the higher boiling point.
Note that C6H12O6 is the formula for glucose, and will not ionize in solution.