Infrared (IR) Spectroscopy - MCAT Physical
Card 0 of 7
An IR spectroscopy reading of a compound shows a large absorption at 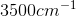 . What is the identity of this compound?
. What is the identity of this compound?
An IR spectroscopy reading of a compound shows a large absorption at 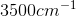
Compounds with an alcohol group show absorptions in an IR sprectrum from  through
through  . Therefore an absorption of
. Therefore an absorption of  would correspond to an alcohol. Amide groups show absorptions from
would correspond to an alcohol. Amide groups show absorptions from  through
through  . Amine groups show absorptions from
. Amine groups show absorptions from  through
through  ,
,  through
through  ,
, 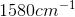 through
through  and
and  through
through  (aromatic amines). Carbonyl compounds such as aldehydes show strong absorptions in the IR spectrum at
(aromatic amines). Carbonyl compounds such as aldehydes show strong absorptions in the IR spectrum at 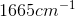 through
through 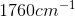 .
.
Compounds with an alcohol group show absorptions in an IR sprectrum from 








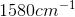



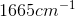
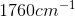
Compare your answer with the correct one above
An IR spectroscopy reading of a compound shows a large absorption at 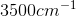 . What is the identity of this compound?
. What is the identity of this compound?
An IR spectroscopy reading of a compound shows a large absorption at 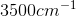
Compounds with an alcohol group show absorptions in an IR sprectrum from  through
through  . Therefore an absorption of
. Therefore an absorption of  would correspond to an alcohol. Amide groups show absorptions from
would correspond to an alcohol. Amide groups show absorptions from  through
through  . Amine groups show absorptions from
. Amine groups show absorptions from  through
through  ,
,  through
through  ,
, 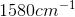 through
through  and
and  through
through  (aromatic amines). Carbonyl compounds such as aldehydes show strong absorptions in the IR spectrum at
(aromatic amines). Carbonyl compounds such as aldehydes show strong absorptions in the IR spectrum at 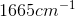 through
through 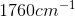 .
.
Compounds with an alcohol group show absorptions in an IR sprectrum from 








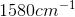



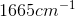
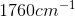
Compare your answer with the correct one above
An IR spectroscopy reading of a compound shows a large absorption at 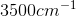 . What is the identity of this compound?
. What is the identity of this compound?
An IR spectroscopy reading of a compound shows a large absorption at 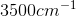
Compounds with an alcohol group show absorptions in an IR sprectrum from  through
through  . Therefore an absorption of
. Therefore an absorption of  would correspond to an alcohol. Amide groups show absorptions from
would correspond to an alcohol. Amide groups show absorptions from  through
through  . Amine groups show absorptions from
. Amine groups show absorptions from  through
through  ,
,  through
through  ,
, 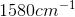 through
through  and
and  through
through  (aromatic amines). Carbonyl compounds such as aldehydes show strong absorptions in the IR spectrum at
(aromatic amines). Carbonyl compounds such as aldehydes show strong absorptions in the IR spectrum at 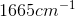 through
through 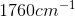 .
.
Compounds with an alcohol group show absorptions in an IR sprectrum from 








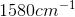



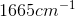
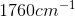
Compare your answer with the correct one above
An IR spectroscopy reading of a compound shows a large absorption at 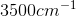 . What is the identity of this compound?
. What is the identity of this compound?
An IR spectroscopy reading of a compound shows a large absorption at 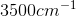
Compounds with an alcohol group show absorptions in an IR sprectrum from  through
through  . Therefore an absorption of
. Therefore an absorption of  would correspond to an alcohol. Amide groups show absorptions from
would correspond to an alcohol. Amide groups show absorptions from  through
through  . Amine groups show absorptions from
. Amine groups show absorptions from  through
through  ,
,  through
through  ,
, 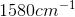 through
through  and
and  through
through  (aromatic amines). Carbonyl compounds such as aldehydes show strong absorptions in the IR spectrum at
(aromatic amines). Carbonyl compounds such as aldehydes show strong absorptions in the IR spectrum at 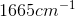 through
through 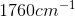 .
.
Compounds with an alcohol group show absorptions in an IR sprectrum from 








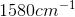



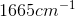
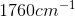
Compare your answer with the correct one above
An IR spectroscopy reading of a compound shows a large absorption at 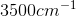 . What is the identity of this compound?
. What is the identity of this compound?
An IR spectroscopy reading of a compound shows a large absorption at 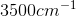
Compounds with an alcohol group show absorptions in an IR sprectrum from  through
through  . Therefore an absorption of
. Therefore an absorption of  would correspond to an alcohol. Amide groups show absorptions from
would correspond to an alcohol. Amide groups show absorptions from  through
through  . Amine groups show absorptions from
. Amine groups show absorptions from  through
through  ,
,  through
through  ,
, 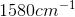 through
through  and
and  through
through  (aromatic amines). Carbonyl compounds such as aldehydes show strong absorptions in the IR spectrum at
(aromatic amines). Carbonyl compounds such as aldehydes show strong absorptions in the IR spectrum at 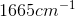 through
through 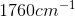 .
.
Compounds with an alcohol group show absorptions in an IR sprectrum from 








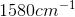



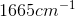
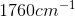
Compare your answer with the correct one above
An IR spectroscopy reading of a compound shows a large absorption at 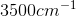 . What is the identity of this compound?
. What is the identity of this compound?
An IR spectroscopy reading of a compound shows a large absorption at 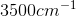
Compounds with an alcohol group show absorptions in an IR sprectrum from  through
through  . Therefore an absorption of
. Therefore an absorption of  would correspond to an alcohol. Amide groups show absorptions from
would correspond to an alcohol. Amide groups show absorptions from  through
through  . Amine groups show absorptions from
. Amine groups show absorptions from  through
through  ,
,  through
through  ,
, 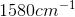 through
through  and
and  through
through  (aromatic amines). Carbonyl compounds such as aldehydes show strong absorptions in the IR spectrum at
(aromatic amines). Carbonyl compounds such as aldehydes show strong absorptions in the IR spectrum at 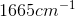 through
through 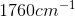 .
.
Compounds with an alcohol group show absorptions in an IR sprectrum from 








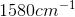



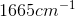
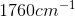
Compare your answer with the correct one above
An IR spectroscopy reading of a compound shows a large absorption at 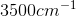 . What is the identity of this compound?
. What is the identity of this compound?
An IR spectroscopy reading of a compound shows a large absorption at 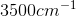
Compounds with an alcohol group show absorptions in an IR sprectrum from  through
through  . Therefore an absorption of
. Therefore an absorption of  would correspond to an alcohol. Amide groups show absorptions from
would correspond to an alcohol. Amide groups show absorptions from  through
through  . Amine groups show absorptions from
. Amine groups show absorptions from  through
through  ,
,  through
through  ,
, 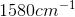 through
through  and
and  through
through  (aromatic amines). Carbonyl compounds such as aldehydes show strong absorptions in the IR spectrum at
(aromatic amines). Carbonyl compounds such as aldehydes show strong absorptions in the IR spectrum at 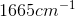 through
through 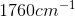 .
.
Compounds with an alcohol group show absorptions in an IR sprectrum from 








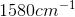



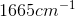
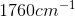
Compare your answer with the correct one above