Substitution and Elimination Mechanisms
Help Questions
MCAT Biology › Substitution and Elimination Mechanisms
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

A scientist is studying the rate of reaction 1. He wants to double the rate of the reaction, but is unsure how to increase concentrations of the reactants. Which of the following is true?
Doubling the concentrations of both the hydroxide and the halide will quadruple the reaction rate
Doubling the concentration of the halide only will quadruple the reaction rate
Doubling the concentration of the hydroxide only will quadruple the reaction rate
Neither doubling the concentration of halide, nor doubling the concentration of hydroxide, will quadruple the reaction rate
Reaction rate in this reaction is not determined by concentration
Explanation
Reaction 1 represents an SN2 reaction. The rate limiting step involves both reactants coming together to form a transition state. The rate of this reaction depends on the concentration of both the organic molecule and the nucleophile.
In contrast, reaction 2 is an E1 reaction, in which the rate limiting step is the removal of the leaving group to form a carbocation. In E1 and SN1 reactions, adjusting the concentration of the halide only is enough to affect the rate.
Which of the following reactions is the nucleophile potassium tert-butoxide often used for?
E2
SN1
SN2
E1
Explanation
Tert-butoxide is a large, sterically hindered, strong nucleophile that is often used in E2 reactions. Strong nucleophiles usually undergo the SN2 or E2 pathway, but tert-butoxide is much too large to undergo a substitution reaction.
Which of the following factors do NOT favor an SN2 reaction of an alkyl halide?
A tertiary carbocation
A primary halide
A good nucleophile
A polar aprotic solvent
Explanation
The way the question is phrased, three answer choices must favor an SN2 reaction, while the "correct" answer is a factor that does not favor, or disfavors an SN2 reaction.
SN2 reactions are bimolecular, and thus their rate of reaction depends on both the substrate and the nucleophile, forming a high energy transition state in which the nucleophile will displace the substate's leaving group at an angle of 180o. The more sterically hindered the compound is, the higher in energy the transition state will be, and the slower the rate of reaction will be. Consequently, SN2 reactions are favored when the leaving group (a halogen in this case) is on a primary carbon center. Additionally, because the reaction is bimolecular, step two of the reaction will NOT occur without a good nucleophile to displace the leaving group. Finally, all SN2 reactions are favored by polar aprotic solvents.
Because SN2 reactions proceed via a transition state, no carbocation intermediate is formed (that happens in SN1 reactions) and therefore the formation of any carbocation favors an SN1 reaction, not an SN2 reaction.
Which of the following compounds could NEVER undergo an E2 reaction when treated with potassium tert-butoxide?
Benzylbromide
Bromoethane
Cyclopentylbromide
3-methyl-3-iodopentane
Cis-2-bromo-1-methylcyclohexane
Explanation
For an E2 reaction to occur, there must be a hydrogen on the carbon adjacent to the carbon with the leaving group. Benzyl bromide contains no hydrogens on the carbon next to the carbon with the bromide, and would therefore undergo only a substitution reaction.
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

Investigating reaction 2, you find that the reaction is initiated when a carbocation forms. Which of the following is likely true?
I. Concentration of the halide is the main determinant of reaction rate
II. The carbocation forms when the hydroxide removes the chlorine atom
III. The carbocation is planar
I and III
I, only
III, only
II and III
I and II
Explanation
The carbocation forms spontaneously with the loss of the chlorine atom. This is the rate determining step, thus, the concentration of the halide is the most important determinant of reaction rate. Carbocations form spontaneously in these reactions, and do not use the strong base to remove the halogen.
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

A scientist modifies reaction 1 by changing the reactant, removing a hydrogen from the central carbon and replacing it with a methyl group. The new reactant thus has two methyl groups and one hydrogen on the central carbon. What is true of reaction 1 following this modification? Assume the temperature remains constant and no catalyst is added.
Reaction 1 proceeds more slowly, owing to a higher activation energy
Reaction 1 proceeds more quickly, owing to a more stable carbocation
Reaction 1 proceeds more slowly, owing to a less stable carbocation
Reaction 1 proceeds more quickly, owing to a decrease in steric hindrance
Reaction 1 only proceeds with a stronger nucleophile
Explanation
Reaction 1 will experience greater steric hindrance with the addition of a methyl group, in place of a hydrogen, on the central carbon of the reactant. The result of this is increased activation energy, and a reduced rate of reaction in unchanging temperature and with no addition of a catalyst.
When exposed to a good nucleophile, which molecule will most readily undergo an 
Explanation

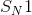

Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

In reaction 2, which of the following describe the rate limiting step?
I. It involves the formation of carbocation
II. It is favored by the presence of substituents on the central carbon
III. It involves a transition state, but no intermediate
I and II
I, only
II, only
III, only
II and III
Explanation
Reaction 2 represents an E1 reaction. The rate limiting step of reaction 2 involves the formation of a carbocation, whose stability is favored by the presence of substituents on the carbon involved. Carbocations are considered intermediates due to their relative stability compared to transition states.
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

The reaction depicted in reaction 1 takes place in solution with a solvent. What type of solvent is most likely to be preferred for the reaction to occur as written?
Polar, aprotic solvent
Polar, protic solvent
Nonpolar, aprotic solvent
Nonpolar, protic solvent
This reaction requires water as a solvent
Explanation
Reaction 1 is an SN2 reaction. This type of substitution reaction prefers a polar, aprotic solvent. The polarity helps to solvate the nucleophile. Aprotic solvents help mediate the transition state and increase reaction rate.
Organic reactions can often be classified into two broad categories: substitution and elimination. Substitution reactions substitute one substituent for another. Elimination reactions typically form after the wholesale removal of a substituent, with no replacement. Below are examples of two types of reactions.
Reaction 1:

Reaction 2:

In reaction 1, a scientist is trying to modify the reaction by using a weaker nucleophile. Which of the following is a weaker nucleophile than what is used above (hydroxide ions)?
Explanation
Nucleophilicity increases to the left on the periodic table. Nucleophilicity will also generally increase with charge. The only equally charged ion in the answers that is present to the right of oxygen on the periodic table is the fluoride ion.








