Amino Acids
Help Questions
MCAT Biology › Amino Acids
A peptide bond is formed between __________.
a carboxyl group and an amino group
two amino groups
two carboxyl groups
two aromatic groups
an ester group and an amine group
Explanation
Each amino acid has an N and a C terminus. The N terminus contains an amino group and the C terminus contains a carboxylic acid group. In order to make a peptide linkage (and eventually create a polypeptide), a bond must form between the amino and carboxylic groups, with water as a byproduct.
Which of the following amino acids is considered basic?
Lysine
Glutamic Acid
Valine
Tyrosine
Explanation
Basic amino acids are those containing an amine group, while acidic contain a carboxylic acid group.
The basic amino acids are lysine (the correct answer), arginine, and histidine.
The acidic amino acids are glutamic acid (glutamate) and aspartic acid (aspartate).
Which of the following amino acids is basic?
Lysine
Proline
Phenylalanine
Alanine
Explanation
On the MCAT you must be able to recognize the following as basic amino acids: lysine, arginine, and histidine. Important acidic amino acids include aspartic acid (aspartate) and glutamic acid (glutamate). Important nonpolar amino acids include: methionine, alanine, isoleucine, proline, phenylalanine, tryptophan, valine, and leucine.
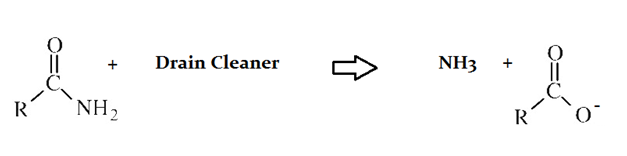
Drain cleaners are a common household staple, used to open clogged drains in bathtubs and sinks. Human hair is a common culprit that clogs pipes, and hair is made predominately of protein. Drain cleaners are effective at breaking down proteins that have accumulated in plumbing. Drain cleaners can be either acidic or basic, and are also effective at breaking down fats that have accumulated with proteins.
A typical reaction—reaction 1—which would be expected for a drain cleaner on contact with human hair, would be as follows in an aqueous solution:
Another reaction that may occur, reaction 2, would take place as follows in an aqueous solution:
In reaction 1, an organic acid forms as a product of the reaction of the original protein and drain cleaner. What quality of the resulting anion contributes most to the acidity of the product?
Resonance stabilization
Polarity of the C-O bond
Electronegativity of the carboxy terminus
Basicity of the side chain
The lone pair of electrons on the original nitrogen
Explanation
The resonance of the two C–O bonds that result after deprotonation of an organic acid is the major contributor to anion stability.
Which two functional groups are included in every amino acid, and take part in amino acids binding together?
Amino group and carboxyl group
Amino group and sulfide group
Sulfide group and carboxyl group
Sulfide group and alcohol
Explanation
Every amino acid contains a carboxyl group and an amino group. These two functional groups are essential for amino acid binding and breaking.
While sulfide groups contribute to higher protein structure by forming disulfide bonds, they do not exist in every amino acid.
All amino acids have at least two pKa values, one corresponding to the carboxylic acid, and one corresponding to the amine functionality. Some amino acids with polar side chains also have a pKa associated with the sidechain functionality.
Phenylalanine has pKa values of 2.58 (carboxylic acid) and 9.24 (NH2).
Arginine has pKa values of 2.01 (carboxylic acid), 9.04 (NH2), and 12.48 (side chain).
Valine has pKa values of 2.29 (carboxylic acid) and 9.72 (NH2).
For this problem, consider a molecule made of up of three amino acids, as described below.
HO-phenylalanine-arginine-valine-NH2
What would the overall charge of this molecule be at a pH of 7?
Explanation
Phenylalanine:
Since the phenylalanine residue is at the C-terminus end of the molecule, only its carboxylic acid pKa is relevant, as its amine is involved in a peptide bond with arginine. At a pH of 7 (well above the carboxylic acid pKa of 2.58), the C-terminus carboxylic acid would be deprotonated and have a charge of 
Arginine:
For the middle amino acid, arginine, the only relevant pKa is that of its side chain since both its carboxylic acid and amino groups are involved in peptide bonds with neighboring amino acids. Since the side chain of arginine would be protonated at a pH of 7 (well below the sidechain pKa of 12.48), this amino acid would have a charge of 
Valine:
Finally, for valine, the relevant pKa to consider is the NH2 pKa of 9.72. At pH 7, this would also be protonated, resulting in a charge of 
The overall charge of the molecule at pH 7 would be 
Which of the following amino acids contain(s) a hydrophilic functional group in its side chain?
I. Serine
II. Valine
III. Phenylalanine
IV. Tyrosine
V. Threonine
I, IV, and V
I and IV
I, II, III, IV, and V
II, III, and IV
Explanation
Serine and threonine are classified as hydrophilic amino acids and contain hydroxyl (-OH) groups in their side chains. Tyrosine, although it is considered hydrophobic, does contain a hydrophilic hydroxyl group in its side chain. The answer is I, IV, and V, as all of these contain hydrophilic functional groups.
A polar amino acid in a highly basic solution is titrated with a strong acid. When will exactly half of the amino acid molecules be negatively charged?
At the first half equivalence point
At the second equivalence point
At the second half equivalence point
At the isoelectric point
Explanation
The amino acid is polar, so we do not need to worry about charges in the side chain. Since the amino acid is starting in a highly basic solution, we know that the amino acid is deprotonated at both termini. That is, the amino terminus is neutral and the carboxyl terminus is negative. This results in a net charge of -1 (at the carboxyl end). The first half equivalence point upon titration will be seen when half of the amino acids are neutral and half of the amino acids are negatively charged. The full first equivalence point will show all molecules with a neutral charge, while the second full equivalence point will show all molecules with a positive charge due to protonation of the amine.
A researcher stains a transmembrane protein. The membrane-spanning region of the protein is stained red, whereas the other regions are stained blue. Which of the following will you most likely find in the red region?
I. Glycine, which has a side chain of
II. Cysteine, which has a side chain of
III. Alanine, which has a side chain of
I and III
I and II
II and III
None of these amino acids will be found in the red region
Explanation
The question states that the membrane-spanning region of the transmembrane protein is stained red; therefore, you will find only hydrophobic amino acids in this region. Recall that an amino acid has a central carbon that has a hydrogen group, carboxylic acid group, amino group, and a side chain attached. The differences between amino acids arise from the different side chains.
A hydrophobic amino acid contains nonpolar side chains. Glycine and alanine contain 


What is the reasoning behind the planar geometry of peptide bonds in proteins?
The carbon-nitrogen bond has partial double bond character
Steric hindrance
Hydrogen bonding
The peptide bond is trans rather than cis
Proteins contain only L amino acids
Explanation
The double bond character of the carbon-nitrogen bond results in a shorter bond compared to the normal length of a pure bond (