Analytical Chemistry - GRE
Card 0 of 384
A chemist carries out the synthetic scheme shown below. Unfortunately, the first two reactions are incomplete, and a mixture of compounds A, B, and C is obtained after the second step. The chemist purifies this mixture by normal phase chromatography, using silica gel as a stationary phase and a 10:1 hexanes-diethyl ether (v:v) solution as an eluent. In what order would compounds A, B, and C elute off the column?
For each choice, the first compound to elute is listed first.

A chemist carries out the synthetic scheme shown below. Unfortunately, the first two reactions are incomplete, and a mixture of compounds A, B, and C is obtained after the second step. The chemist purifies this mixture by normal phase chromatography, using silica gel as a stationary phase and a 10:1 hexanes-diethyl ether (v:v) solution as an eluent. In what order would compounds A, B, and C elute off the column?
For each choice, the first compound to elute is listed first.

In the normal phase chromatography system described, the most nonpolar compound would elute first and the most polar compound would elute last. The silica stationary phase will interact with more polar molecules, while the hexane mobile phase will carry nonpolar molecules. This would slow the progress of polar molecules as they bond to the silica, and enhance the progress of nonpolar molecules as they interact with the mobile phase.
Compound C is the most nonpolar compound because it contains only hydrogen and carbon. Compounds A and B are more polar because of the presence of oxygen, and hence the presence of polarized carbon-oxygen bonds. The alcohol group of compound B makes this compound the most polar of the three molecules by virtue of hydrogen bonding capabilities as well as the carbon-oxygen dipole. Compound B would thus elute last.
In the normal phase chromatography system described, the most nonpolar compound would elute first and the most polar compound would elute last. The silica stationary phase will interact with more polar molecules, while the hexane mobile phase will carry nonpolar molecules. This would slow the progress of polar molecules as they bond to the silica, and enhance the progress of nonpolar molecules as they interact with the mobile phase.
Compound C is the most nonpolar compound because it contains only hydrogen and carbon. Compounds A and B are more polar because of the presence of oxygen, and hence the presence of polarized carbon-oxygen bonds. The alcohol group of compound B makes this compound the most polar of the three molecules by virtue of hydrogen bonding capabilities as well as the carbon-oxygen dipole. Compound B would thus elute last.
Compare your answer with the correct one above
Which of the following functional groups would be expected to have the largest  value during a thin-layer chromatography (TLC) experiment with an ether solvent?
value during a thin-layer chromatography (TLC) experiment with an ether solvent?
Which of the following functional groups would be expected to have the largest 
The  value is proportional to the affinity of the solute to the solvent. The solvent acts as the mobile phase along a polar paper stationary phase. Polar compounds will interact more with the paper, travelling slowly, while nonpolar compounds will interact more with the solvent, travelling more quickly.
value is proportional to the affinity of the solute to the solvent. The solvent acts as the mobile phase along a polar paper stationary phase. Polar compounds will interact more with the paper, travelling slowly, while nonpolar compounds will interact more with the solvent, travelling more quickly.
A large  value represents a large proclivity for the mobile solvent in the experiment. Because we are using ether, a non-polar solvent, we would expect non-polar compounds to travel the farthest on our plate. Of the answer choices, alkanes are the least polar and would thus travel farthest into the mobile phase of the four functional groups. A polar functional group, like a halide, will interact more in the stationary phase, and will thus has a significantly smaller
value represents a large proclivity for the mobile solvent in the experiment. Because we are using ether, a non-polar solvent, we would expect non-polar compounds to travel the farthest on our plate. Of the answer choices, alkanes are the least polar and would thus travel farthest into the mobile phase of the four functional groups. A polar functional group, like a halide, will interact more in the stationary phase, and will thus has a significantly smaller  value.
value.
The 
A large 

Compare your answer with the correct one above
Which of the following purification techniques would best separate a nonpolar solute from a polar solute?
Which of the following purification techniques would best separate a nonpolar solute from a polar solute?
Generally, extraction is the best means of separating two solutes based on polarity. This technique allows separation based on solubility in two different solvents, which separate based on polarity.
Extraction, however, is not offered as an answer. The next best option would be thin layer chromatography. In this process, a polar stationary phase is introduced to a nonpolar solvent. Solutes are placed on the stationary phase. The nonpolar solvent acts as the mobile phase. Nonpolar solvents interact more with the mobile solvent, travelling quickly along the polar stationary phase, while polar solutes are attracted to the stationary phase and travel more slowly. This property allows for separation based on polarity.
Ion exchange chromatography is used to separate compounds with different charges, not necessarily differing polarities. Mass spectroscopy will identify compounds based on mass, and distillation will allow for separation based on differences in boiling point and vapor pressure.
Generally, extraction is the best means of separating two solutes based on polarity. This technique allows separation based on solubility in two different solvents, which separate based on polarity.
Extraction, however, is not offered as an answer. The next best option would be thin layer chromatography. In this process, a polar stationary phase is introduced to a nonpolar solvent. Solutes are placed on the stationary phase. The nonpolar solvent acts as the mobile phase. Nonpolar solvents interact more with the mobile solvent, travelling quickly along the polar stationary phase, while polar solutes are attracted to the stationary phase and travel more slowly. This property allows for separation based on polarity.
Ion exchange chromatography is used to separate compounds with different charges, not necessarily differing polarities. Mass spectroscopy will identify compounds based on mass, and distillation will allow for separation based on differences in boiling point and vapor pressure.
Compare your answer with the correct one above
Chromatography involves the separation of a mixture by allowing a mobile phase to travel along a stationary phase. In thin layer chromatography (TLC), a liquid solution is able to travel along a stationary plate. The distance that a particular compound travels compared to another compound can be determined by comparing the Rf factors for each compound. The Rf factor is determined by dividing the compound's distance by the total distance of the solvent.
Which of the following compounds would have the smallest Rf factor in a standard thin-layer chromatography (TLC) experiment?
Chromatography involves the separation of a mixture by allowing a mobile phase to travel along a stationary phase. In thin layer chromatography (TLC), a liquid solution is able to travel along a stationary plate. The distance that a particular compound travels compared to another compound can be determined by comparing the Rf factors for each compound. The Rf factor is determined by dividing the compound's distance by the total distance of the solvent.
Which of the following compounds would have the smallest Rf factor in a standard thin-layer chromatography (TLC) experiment?
The stationary phase in chromatography is typically attracted to the more polar compounds in a solution, while the mobile phase carried the nonpolar compounds. As a result, more polar compounds will move a shorter distance, resulting in a lower Rf factor. Glucose is a very polar molecule, and would move a shorter distance compared to the other options.
The stationary phase in chromatography is typically attracted to the more polar compounds in a solution, while the mobile phase carried the nonpolar compounds. As a result, more polar compounds will move a shorter distance, resulting in a lower Rf factor. Glucose is a very polar molecule, and would move a shorter distance compared to the other options.
Compare your answer with the correct one above
DNA is negatively charged, so it will migrate toward the positive electrode during electrophoresis. As a result, it will migrate from the center line into the green region of the gel.
DNA is negatively charged, so it will migrate toward the positive electrode during electrophoresis. As a result, it will migrate from the center line into the green region of the gel.
Compare your answer with the correct one above
When would it be appropriate to use extraction in order to separate compounds in a solution?
When would it be appropriate to use extraction in order to separate compounds in a solution?
Extraction is a useful separation technique when there is a mixture of compounds in a solution that have similar polarities, but different solubilities. The three-step process of extraction can take advantage of different solubilities by introducing the mixture to different acidic and basic conditions.
Extraction is a useful separation technique when there is a mixture of compounds in a solution that have similar polarities, but different solubilities. The three-step process of extraction can take advantage of different solubilities by introducing the mixture to different acidic and basic conditions.
Compare your answer with the correct one above
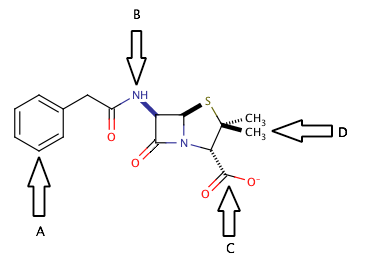
The correct answer is arrow C. Separation into the aqueous layer during liquid-liquid extraction is most easily accomplished by creating a salt somewhere in the molecule. There are two common targets for creating salts in organic molecules. The first target is to create a carboxylate salt, by the addition of an inorganic base (such as NaOH) to a carboxylic acid or an existing carboxylate group, as shown in the diagram at arrow C.
Another common target for forming a salt would be to protonate the nitrogens of the molecule. Addition of an inorganic acid (such as HCl) would be a good way to protonate the nitrogens and also provide a negatively charged ion that would form the salt. In this case, however, the nitrogen at arrow B is involved in an amide functional group, and cannot be protonated. For the nitrogen to be useful in extraction, it must be part of an amine group.
The main point to take away from here is that during liquid-liquid extraction, the goal is to separate compounds into aqueous and organic layers and isolate the desired product alone in one of these layers. Ions and salts will generally be extracted into the aqueous layer, where nonpolar large structures will generally be extracted into the organic layer. Look for areas on the molecule that are susceptible to acid/base reactions, and you will find a good target for creating an ion for liquid-liquid extraction.
The correct answer is arrow C. Separation into the aqueous layer during liquid-liquid extraction is most easily accomplished by creating a salt somewhere in the molecule. There are two common targets for creating salts in organic molecules. The first target is to create a carboxylate salt, by the addition of an inorganic base (such as NaOH) to a carboxylic acid or an existing carboxylate group, as shown in the diagram at arrow C.
Another common target for forming a salt would be to protonate the nitrogens of the molecule. Addition of an inorganic acid (such as HCl) would be a good way to protonate the nitrogens and also provide a negatively charged ion that would form the salt. In this case, however, the nitrogen at arrow B is involved in an amide functional group, and cannot be protonated. For the nitrogen to be useful in extraction, it must be part of an amine group.
The main point to take away from here is that during liquid-liquid extraction, the goal is to separate compounds into aqueous and organic layers and isolate the desired product alone in one of these layers. Ions and salts will generally be extracted into the aqueous layer, where nonpolar large structures will generally be extracted into the organic layer. Look for areas on the molecule that are susceptible to acid/base reactions, and you will find a good target for creating an ion for liquid-liquid extraction.
Compare your answer with the correct one above
A student has just finished running a reaction for organic chemistry lab. The reaction has produced a primary amine, as well as multiple dialkyl ether byproducts. The student has 1N aqueous HCl, 1N aqueous NaOH, water, and ethyl acetate at his disposal for purification. After diluting the crude reaction mixture with ethyl acetate, which of the following extraction methods will successfully isolate the amine product?
A student has just finished running a reaction for organic chemistry lab. The reaction has produced a primary amine, as well as multiple dialkyl ether byproducts. The student has 1N aqueous HCl, 1N aqueous NaOH, water, and ethyl acetate at his disposal for purification. After diluting the crude reaction mixture with ethyl acetate, which of the following extraction methods will successfully isolate the amine product?
To isolate the amine, it is first necessary to treat it with acid to form an ammonium salt. This would go into the aqueous phase along with the HCl, while the dialkyl ether byproducts would remain behind in the organic phase of the crude mixture.
After discarding the organic phase, treating the aqueous phase with 1N NaOH would convert the ammonium salt back to the free amine, which could then be extracted with ethyl acetate.
As for the other answers, extracting the organic phase with aqueous NaOH would leave the amine behind in the organic phase, as would washing with water. Also, the protonated amine would have to be neutralized in the aqueous phase with NaOH in order for it to be extracted back into ethyl acetate.
To isolate the amine, it is first necessary to treat it with acid to form an ammonium salt. This would go into the aqueous phase along with the HCl, while the dialkyl ether byproducts would remain behind in the organic phase of the crude mixture.
After discarding the organic phase, treating the aqueous phase with 1N NaOH would convert the ammonium salt back to the free amine, which could then be extracted with ethyl acetate.
As for the other answers, extracting the organic phase with aqueous NaOH would leave the amine behind in the organic phase, as would washing with water. Also, the protonated amine would have to be neutralized in the aqueous phase with NaOH in order for it to be extracted back into ethyl acetate.
Compare your answer with the correct one above
A first-year graduate student treats compound A (below) with aqueous sodium hydroxide and heats the reaction for one hour. After cooling down the reaction, what must he do to isolate the desired carboxylic acid product?

A first-year graduate student treats compound A (below) with aqueous sodium hydroxide and heats the reaction for one hour. After cooling down the reaction, what must he do to isolate the desired carboxylic acid product?

During the reaction, the ester is converted to a carboxylic acid, however, because of the basic conditions the acid would be deprotonated to form a carboxylate salt. This salt would be soluble in the aqueous phase, and would have to be protonated by adding dilute acid to convert it into a neutral molecule. We want to isolate the carboxylic acid product via extraction, forcing it from the aqueous phase to the organic phase. Only when the product is neutral can it be extracted by organic ethyl acetate.
Simply concentrating the reaction mixture, either by itself or after neutralizing any base with acid, would be insufficient because residual inorganic salts would remain with the desired carboxylic acid. Likewise, extracting the reaction with ethyl acetate without adding any acid would not extract the product, as it would remain in the aqueous phase as a carboxylate salt.
During the reaction, the ester is converted to a carboxylic acid, however, because of the basic conditions the acid would be deprotonated to form a carboxylate salt. This salt would be soluble in the aqueous phase, and would have to be protonated by adding dilute acid to convert it into a neutral molecule. We want to isolate the carboxylic acid product via extraction, forcing it from the aqueous phase to the organic phase. Only when the product is neutral can it be extracted by organic ethyl acetate.
Simply concentrating the reaction mixture, either by itself or after neutralizing any base with acid, would be insufficient because residual inorganic salts would remain with the desired carboxylic acid. Likewise, extracting the reaction with ethyl acetate without adding any acid would not extract the product, as it would remain in the aqueous phase as a carboxylate salt.
Compare your answer with the correct one above
Extraction is the process of removing compounds from a solution by taking advantage of varying solubilities. The process of extraction for a mixture of diethylamine, phenol, acetic acid, and ammonia involves three steps.
1. Add a strong acid and shake the solution. Let the aqueous layer drain off.
2. Add a weak base and shake the solution. Let the aqueous layer drain off.
3. Add a strong base and shake the solution. Let the aqueous layer drain off.
Which of the following compounds would be the last to enter the aqueous phase using this technique?
Extraction is the process of removing compounds from a solution by taking advantage of varying solubilities. The process of extraction for a mixture of diethylamine, phenol, acetic acid, and ammonia involves three steps.
1. Add a strong acid and shake the solution. Let the aqueous layer drain off.
2. Add a weak base and shake the solution. Let the aqueous layer drain off.
3. Add a strong base and shake the solution. Let the aqueous layer drain off.
Which of the following compounds would be the last to enter the aqueous phase using this technique?
Think about what will be reacting with each substance as it is introduced to the solution.
First, a strong acid is added. This will make all bases in the solution (ammonia and diethylamine) become protonated, allowing them to become soluble. They will transition to the aqueous phase and be removed.
Next, a weak base is added. Only carboxylic acids will be deprotonated by this addition, gaining a charge and transitioning to the aqueous phase. Acetic acid will be extracted.
Finally, a strong base will deprotonate the weak acids, such as phenol. As a result, phenol will be the last molecule to leave the solution.
Think about what will be reacting with each substance as it is introduced to the solution.
First, a strong acid is added. This will make all bases in the solution (ammonia and diethylamine) become protonated, allowing them to become soluble. They will transition to the aqueous phase and be removed.
Next, a weak base is added. Only carboxylic acids will be deprotonated by this addition, gaining a charge and transitioning to the aqueous phase. Acetic acid will be extracted.
Finally, a strong base will deprotonate the weak acids, such as phenol. As a result, phenol will be the last molecule to leave the solution.
Compare your answer with the correct one above
Which of the following mixtures would not be adequately separated via distillation?
Which of the following mixtures would not be adequately separated via distillation?
Distillation involves the separation of compounds by taking advantage of their different boiling points. Mixtures with similar boiling points would not be adequately separated using distillation.
A racemic mixture is a mixture of enantiomers, which have identical boiling points. As a result, distillation would not be effective for separating a racemic mixture.
Diastereomers will have differing physical properties and can be separated by boiling point. Larger alkane chains result in higher boiling points, allowing separation of different sized alcohols by distillation. Polar compounds will have greater intermolecular forces, generally leading to higher boiling points, and allowing them to be separated from nonpolar compounds.
Distillation involves the separation of compounds by taking advantage of their different boiling points. Mixtures with similar boiling points would not be adequately separated using distillation.
A racemic mixture is a mixture of enantiomers, which have identical boiling points. As a result, distillation would not be effective for separating a racemic mixture.
Diastereomers will have differing physical properties and can be separated by boiling point. Larger alkane chains result in higher boiling points, allowing separation of different sized alcohols by distillation. Polar compounds will have greater intermolecular forces, generally leading to higher boiling points, and allowing them to be separated from nonpolar compounds.
Compare your answer with the correct one above
Michael's scale measures the mass of objects as consistently  less than their actual mass. How would you describe the scale?
less than their actual mass. How would you describe the scale?
Michael's scale measures the mass of objects as consistently 
Precision measures is how consistently a device records the same answer. In this case, Michael's scale is ALWAYS  short. Even though it displays the wrong value, it is consistent. That means it is precise. Measuring a
short. Even though it displays the wrong value, it is consistent. That means it is precise. Measuring a  object will always display a mass of
object will always display a mass of  ; the results are easily reproduced.
; the results are easily reproduced.
Accuracy is how well a device measures something against its accepted value. In this case, Michael's scale is not accurate because it is always off by  .
.
Precision measures is how consistently a device records the same answer. In this case, Michael's scale is ALWAYS 


Accuracy is how well a device measures something against its accepted value. In this case, Michael's scale is not accurate because it is always off by 
Compare your answer with the correct one above
Michael buys several bags of balloons. On the package, it says that each bag has 100 balloons. He opens the bags and only one of them has 100 balloons inside; the other bags either have too many or too few.
How would you describe the bag of balloons with 100 balloons inside?
Michael buys several bags of balloons. On the package, it says that each bag has 100 balloons. He opens the bags and only one of them has 100 balloons inside; the other bags either have too many or too few.
How would you describe the bag of balloons with 100 balloons inside?
This bag is accurate because it provided the correct number of balloons, however, the process is not precise as the results were clearly not repeatable.
Accuracy deals with how close the measurement got to the accepted measurement. Precision deals with how consistent the measurement is. The bag with 100 balloons inside matched the claim made on the bag, meaning it was accurate. It was not precise because the other measurements show that the number of balloons is variable.
This bag is accurate because it provided the correct number of balloons, however, the process is not precise as the results were clearly not repeatable.
Accuracy deals with how close the measurement got to the accepted measurement. Precision deals with how consistent the measurement is. The bag with 100 balloons inside matched the claim made on the bag, meaning it was accurate. It was not precise because the other measurements show that the number of balloons is variable.
Compare your answer with the correct one above
An brand of fruit snacks claims that each bag of fruit snacks has a mass of  . After weighing three bags, Wally observes the masses to be
. After weighing three bags, Wally observes the masses to be  ,
,  , and
, and  .
.
How can Wally describe the accuracy and precision of the first bag he measured?
An brand of fruit snacks claims that each bag of fruit snacks has a mass of 



How can Wally describe the accuracy and precision of the first bag he measured?
The claim for the mass of the first bag is accurate; the brand says there should be  in each bag and there was
in each bag and there was  in the first bag.
in the first bag.
The claim on the first bag is not precise, as the results are not replicated universally throughout the experiment. The masses of the bags fluctuate, with the average of all three bags equal to  .
.
The claim for the mass of the first bag is accurate; the brand says there should be 

The claim on the first bag is not precise, as the results are not replicated universally throughout the experiment. The masses of the bags fluctuate, with the average of all three bags equal to 
Compare your answer with the correct one above
A bowman is shooting arrows at a target. Which of the following demonstrates high accuracy but low precision?
A bowman is shooting arrows at a target. Which of the following demonstrates high accuracy but low precision?
Accuracy is measured as the degree of closeness to the actual measurement. In our case, accurate shots will hit the bullseye. Precision is measured as the degree of closeness of one measurement to the next. In our case, precise shots will be clustered together.
To get high accuracy but low precision, measurements must center around the target value but be variable. The bowman's arrows will not be clustered (low precision), but will be accurately distributed around the bullseye. If all the shots were averaged, the bullseye would be at the center.
Accuracy is measured as the degree of closeness to the actual measurement. In our case, accurate shots will hit the bullseye. Precision is measured as the degree of closeness of one measurement to the next. In our case, precise shots will be clustered together.
To get high accuracy but low precision, measurements must center around the target value but be variable. The bowman's arrows will not be clustered (low precision), but will be accurately distributed around the bullseye. If all the shots were averaged, the bullseye would be at the center.
Compare your answer with the correct one above
Which of these is an example of high accuracy?
Which of these is an example of high accuracy?
Accuracy is the measure of difference between a calculated value and the true value of a measurement. High accuracy demands that the experimental result be equal to the theoretical result.
In contrast, precision is a measure of reproducibility. If multiple trials produce the same result each time with minimal deviation, then the experiment has high precision. This is true even if the results are not true to the theoretical predictions; an experiment can have high precision with low accuracy.
An archer hitting a bulls-eye is an example of high accuracy, while an archer hitting the same spot on the bulls-eye three times would be an example of high precision.
Accuracy is the measure of difference between a calculated value and the true value of a measurement. High accuracy demands that the experimental result be equal to the theoretical result.
In contrast, precision is a measure of reproducibility. If multiple trials produce the same result each time with minimal deviation, then the experiment has high precision. This is true even if the results are not true to the theoretical predictions; an experiment can have high precision with low accuracy.
An archer hitting a bulls-eye is an example of high accuracy, while an archer hitting the same spot on the bulls-eye three times would be an example of high precision.
Compare your answer with the correct one above
Which of these is an example of high precision?
Which of these is an example of high precision?
Precision is a measure of reproducibility. If multiple trials produce the same result each time with minimal deviation, then the experiment has high precision. This is true even if the results are not true to the theoretical predictions; an experiment can have high precision with low accuracy.
In contrast, accuracy is the measure of difference between a calculated value and the true value of a measurement. High accuracy demands that the experimental result be equal to the theoretical result.
An archer hitting a bulls-eye is an example of high accuracy, while an archer hitting the same spot on the bulls-eye three times would be an example of high precision.
Precision is a measure of reproducibility. If multiple trials produce the same result each time with minimal deviation, then the experiment has high precision. This is true even if the results are not true to the theoretical predictions; an experiment can have high precision with low accuracy.
In contrast, accuracy is the measure of difference between a calculated value and the true value of a measurement. High accuracy demands that the experimental result be equal to the theoretical result.
An archer hitting a bulls-eye is an example of high accuracy, while an archer hitting the same spot on the bulls-eye three times would be an example of high precision.
Compare your answer with the correct one above
What is the molar concentration of a 2% by mass  solution with a density of
solution with a density of  ?
?
What is the molar concentration of a 2% by mass 

By definition, 2% by mass means 2 grams of  in every 100 grams of solution. In order to solve this problem, we may assume we are dealing with 100 grams of solution. We can use the density as a conversion factor to determine the volume of solution we are dealing with:
in every 100 grams of solution. In order to solve this problem, we may assume we are dealing with 100 grams of solution. We can use the density as a conversion factor to determine the volume of solution we are dealing with:


In order to calculate the number of moles of  , we need to convert 2 grams of
, we need to convert 2 grams of  to moles using it's molecular weight. This is because we are assuming we are dealing 100 grams of a 2%
to moles using it's molecular weight. This is because we are assuming we are dealing 100 grams of a 2%  solution.
solution.

In order to calculate molarity, we need to plug the moles and volumes calculated into the following equation:

By definition, 2% by mass means 2 grams of 
In order to calculate the number of moles of 


In order to calculate molarity, we need to plug the moles and volumes calculated into the following equation:
Compare your answer with the correct one above

Using the calibration curve given, if given the absorbance of 1.51, what would be the concentration?

Using the calibration curve given, if given the absorbance of 1.51, what would be the concentration?
A calibration curve is a graph that gives the relationship between a measured quantity and an analytical signal. In this question the analytical signal is absorbance and the measured quantity is the concentration. To answer this problem, simply determine where the absorbance value 1.5 is on the y-axis and find what point the x-axis crosses this absorbance.
A calibration curve is a graph that gives the relationship between a measured quantity and an analytical signal. In this question the analytical signal is absorbance and the measured quantity is the concentration. To answer this problem, simply determine where the absorbance value 1.5 is on the y-axis and find what point the x-axis crosses this absorbance.
Compare your answer with the correct one above
A researcher performs a Bradford assay to determine the quantity of an unknown protein in his sample. The standard protein returns absorbance values of 0.101, 0.204, 0.302, 0.405 for the respective quantities of 10ug, 20ug, 30ug, and 40ug of protein. The unknown sample returns an absorbance value of 0.265. What is the quantity of protein in the unknown sample?
A researcher performs a Bradford assay to determine the quantity of an unknown protein in his sample. The standard protein returns absorbance values of 0.101, 0.204, 0.302, 0.405 for the respective quantities of 10ug, 20ug, 30ug, and 40ug of protein. The unknown sample returns an absorbance value of 0.265. What is the quantity of protein in the unknown sample?
For this problem we need to determine the equation of our standard curve. This can be done by creating a graph from the data points and determining the slope.

Assuming that a sample of zero concentration will also have zero absorbance, we can find the equation for the line generated by finding the slope.


Pick two points on the line to find the slope. We will use (0,0) and (40,0.405).


Use this equation and the absorbance given in the question to find the concentration of the unknown sample.


For this problem we need to determine the equation of our standard curve. This can be done by creating a graph from the data points and determining the slope.

Assuming that a sample of zero concentration will also have zero absorbance, we can find the equation for the line generated by finding the slope.
Pick two points on the line to find the slope. We will use (0,0) and (40,0.405).
Use this equation and the absorbance given in the question to find the concentration of the unknown sample.
Compare your answer with the correct one above