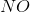0%
0 / 1 answered
Equilibrium Constant Practice Test
•1 QuestionsQuestion
1 / 1
Q1
}$$+2H_{2 (g)}$\leftrightharpoons $N_{2 (g)}$$+2H_{2}$$O_{(g)}$")
In the above reaction, by what factor would the reaction quotient change if the concentration of  were doubled?
were doubled?
}$$+2H_{2 (g)}$\leftrightharpoons $N_{2 (g)}$$+2H_{2}$$O_{(g)}$")
In the above reaction, by what factor would the reaction quotient change if the concentration of