Titrations
Help Questions
GRE Subject Test: Chemistry › Titrations
Considering the given chemical reaction, determine the number of moles of 

Explanation




Which of the following is an indication of when a reaction has reached the equivalence point.
Moles of titrant equals moles of analyte
The end point of a titration
When the volume of both reactants are equal
When there is a color change
Explanation
The equivalence point is the point in a titration at which the number of moles of titrant equals the number of moles of analyte. The endpoint in a titration is when the color of the reaction changes to that of the desired (often neutral) pH. However this is not the same as the equivalence point, which is a stoichiometric value. Indicators may change colors several times throughout a neutralization reaction, thus this is not an accurate attribute of the reaction to use as the equivalence point. Furthermore, the concentrations of titrant and the analyte are rarely equivalent, and there could be differing numbers of ionizable groups on each species.
A 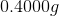



Explanation
A pH indicator added to a solution causes a color change which is an indication that a reaction has reached the equivalence point. The equivalence point is when the number of moles of titrant equals the number of moles of analyte. The end point is an approximation of this in a titration.
At the equivalence point:
The number of moles of NaOH at equivalence point is calculated as follows:
Assuming we were trying to convert the number of grams of the monoprotic acid to moles, the equation would be set up as follows:
Let's plug the values we have into this equation and give the molar mass a value of 
Rearranging this equation to solve for the molar mass 

Based on the pH titration curve provided, how many acidic protons are in this acid?
Two
One
Three
Four
It cannot be determined from the graph
Explanation
The equivalence point is the point in a titration at which the number of moles of titrant species equals the number of moles of analyte. For a polyprotic acid there are multiple equivalence points because there are more than one acidic proton in one molecule of the acid. A titration curve has as many equivalence points as the number of protons that may be neutralized by the interaction with a base. In this titration curve there are two equivalence points. The equivalence points occur when the graph has a very steep slope, and involve very large changes in pH with additions of small amounts of base. For this graph, the equivalence points occur at about pH 3.5 and 10.
Considering the given neutralization reaction, what is the concentration of the sodium chloride solution if it takes 20.00mL of 0.045M 

Explanation
Based on the equation given, we know the that 



To determine the molar concentration of 
We have determined that the concentration of 

A vinegar sample was determined by a titration with 


Explanation
We must first calculate the molecular weight of 
We can calculate the grams of 
Finally, we can calculate the percent mass using the total grams of vinegar solution:
Which of the following pH values is an acceptable equivalence point for a weak base being titrated by a strong acid?
Explanation
The equivalence point is the point during a titration when there are equal equivalents of acid and base in the solution. Since a strong acid will have more effect on the pH than the same amount of a weak base, we predict that the solution's pH will be acidic at the equivalence point. 5.2 and 1.3 are both acidic, but 1.3 is remarkably acidic considering that there is an equal amount of base in the solution. As a result, 5.2 is a more appropriate answer.
The equivalence point for a strong acid and strong base will be 7.0. When one of the compounds is weak, however, it will dissociate less than its strong counterpart. In our case, the base will dissociate less than the acid. The acid, thus, contributes more to the pH character of the solution.
A weak acid is slowly titrated with a strong base. Where on the titration curve would the solution be the most well-buffered?
The half equivalence point
The equivalence point
When no base has been added
When the amount of base added equals the amount of acid in the solution.
Explanation
At the half equivalence point, the concentration of acid in the solution is equal to the concentration of the conjugate base in solution. At this point, the graph shows a line that is near horizontal. This means that base or acid could be added and the pH of the solution would change very slowly.
Remember that pH is on the y-axis of the titration curve; thus a near-horizontal line will signify a region where pH is most stable. At the half equivalence point, pH = pKa.
Considering the given chemical reaction, determine the concentration of 

Explanation




What volume in liters of 




Explanation
The equivalence point in a titration is the point in which the number of moles of titrant equals to the number of moles of analyte:
We need to determine the number of moles of acetic acid we are dealing with:
Therefore, at the equivalence point there are also 0.0075 moles of 



















































